Abstract
1. Respiratory responses were measured in forty-seven cats, made decerebrate or anaesthetized with pentobarbitone or chloralose-urethane, to changes in the level of CO2 infused into the inferior vena cava via an external oxygenator circuit or to changes in the volume of venous return or to the inhalation of CO2.
2. No consistent difference was found between the respiratory response to the increase in the level of CO2 infused or CO2 inhaled provided that the volume of venous return during both sets of tests was held constant at normal levels.
3. If the volume of venous return was increased and the level of CO2 infused maintained at levels such that ˙VCO2 did not increase, ventilation increased with a fall in Pa, CO2, a response lying approximately on the isometabolic curve.
4. If the volume of venous return and the level of CO2 infusion were raised together, a spectrum of intermediate respiratory responses was obtained which reproduced all those seen in earlier papers, in muscular exercise or other hypermetabolic states.
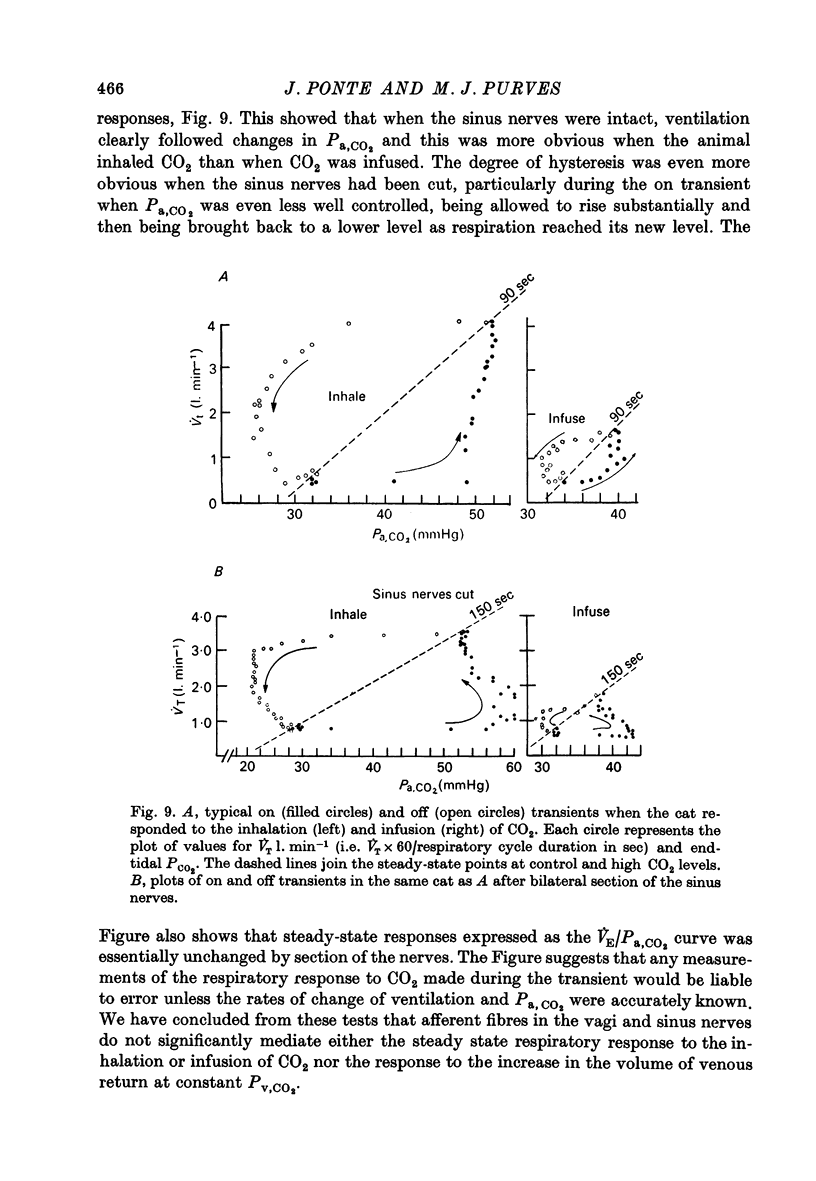
5. None of the steady-state respiratory responses was significantly affected by bilateral vagotomy or section of the sinus nerves, though sinus nerve section slowed the responses to infused or inhaled CO2 and they were then less precisely controlled.
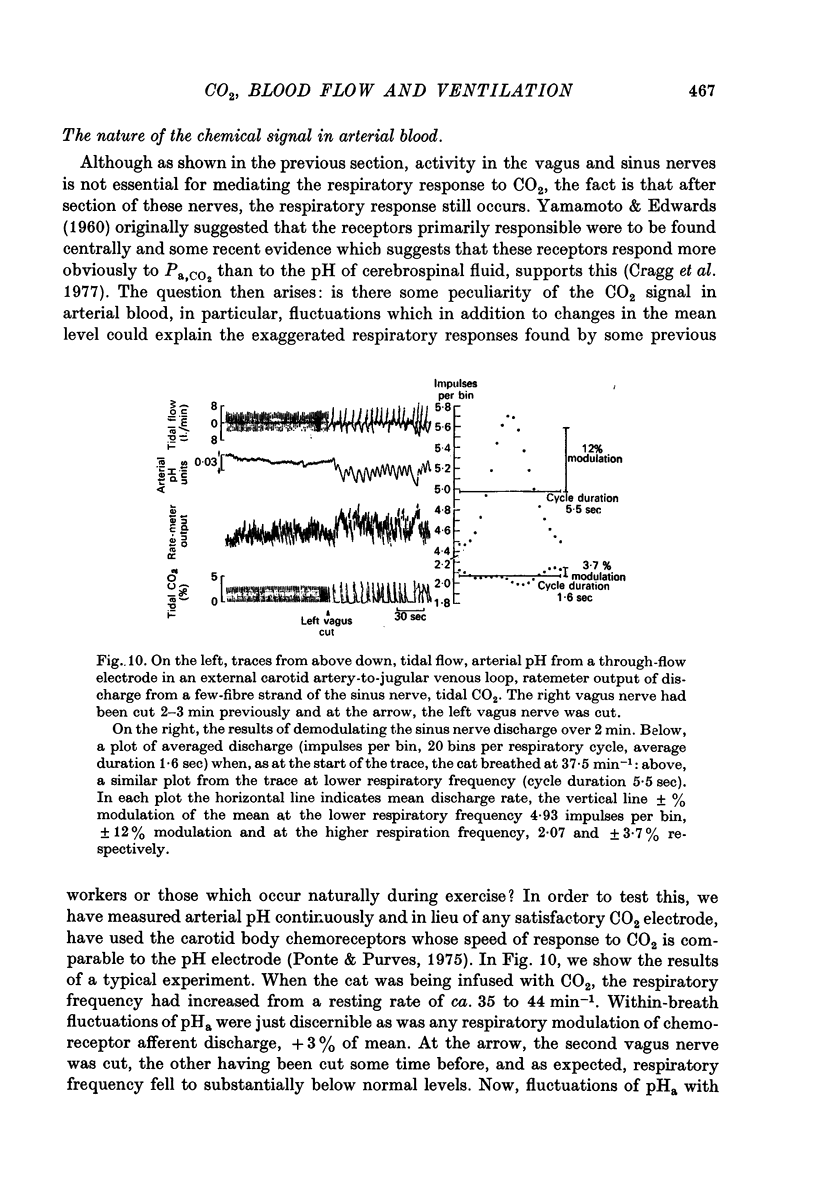
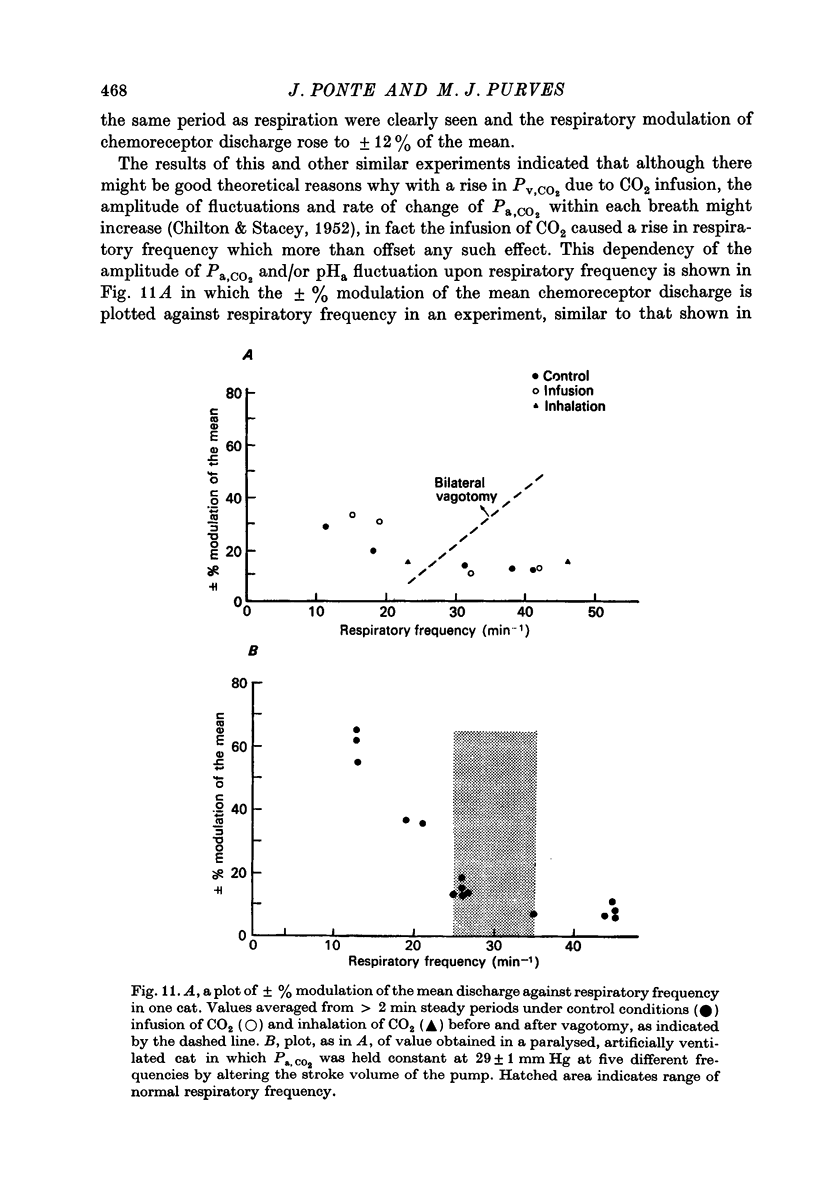
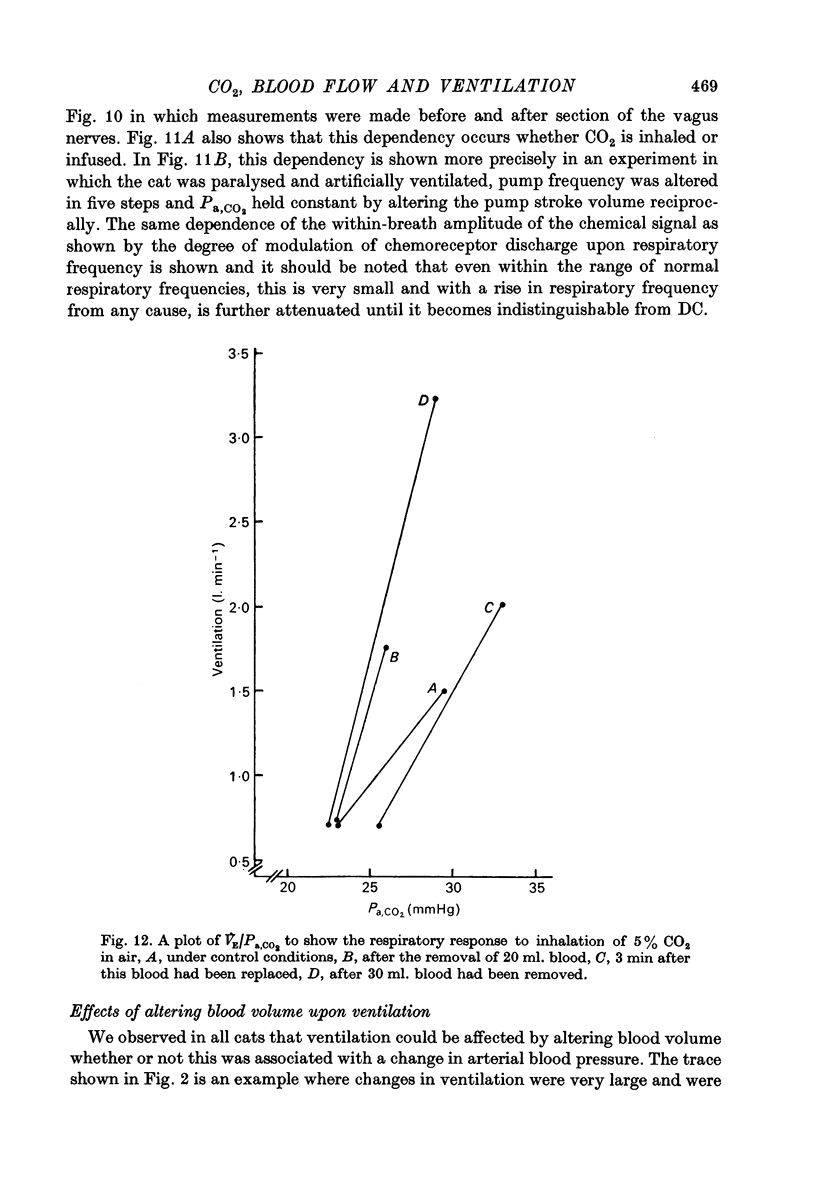
6. Additional experiments indicated that where CO2 was infused or inhaled, the effective stimulus to respiration was an increase in mean Pa, CO2 in proportion to the CO2 added and that the respiratory response to CO2 was enhanced by reduced blood volume. How the changes in venous return were sensed and affected respiration, remains unclear.
7. These results may explain why previous workers have obtained exaggerated respiratory responses to the infusion of CO2 and why respiration increases rapidly at the start of exercise and is then maintained at high levels without discernible change in the chemical stimulus in arterial blood.
Full text
PDF

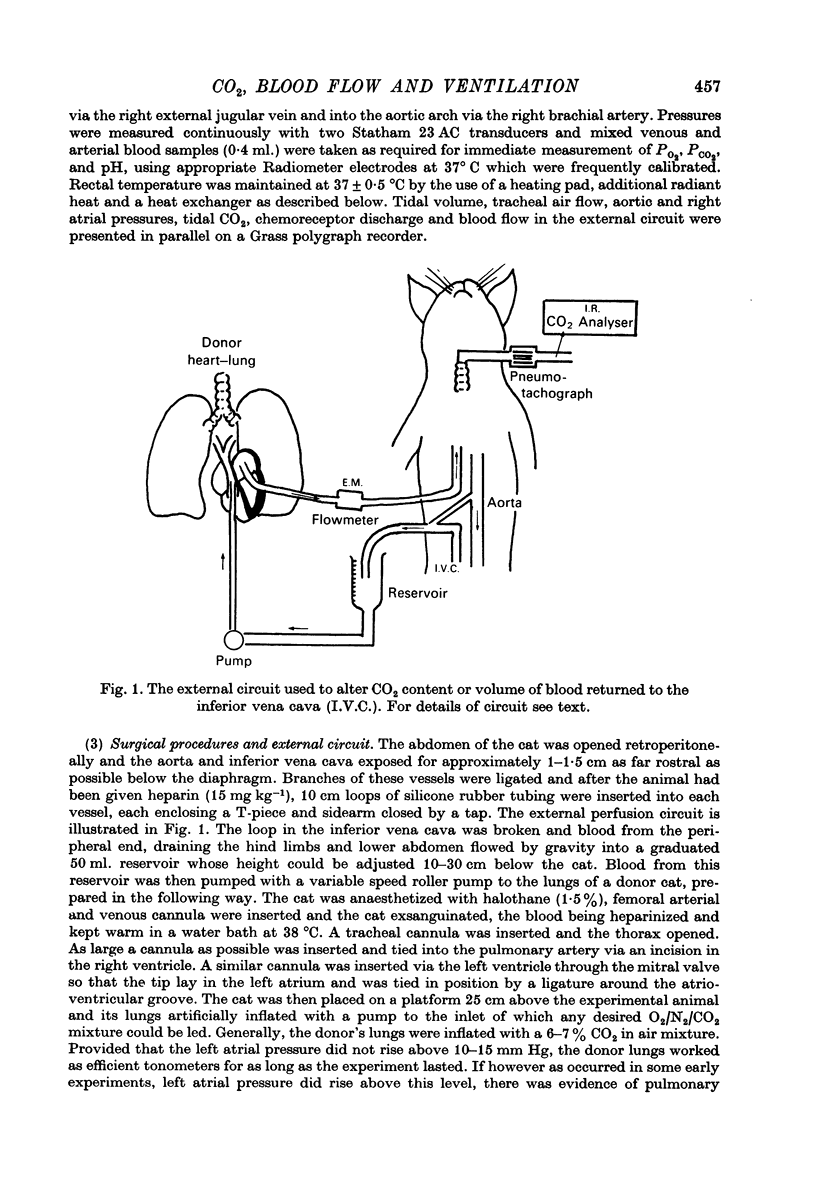
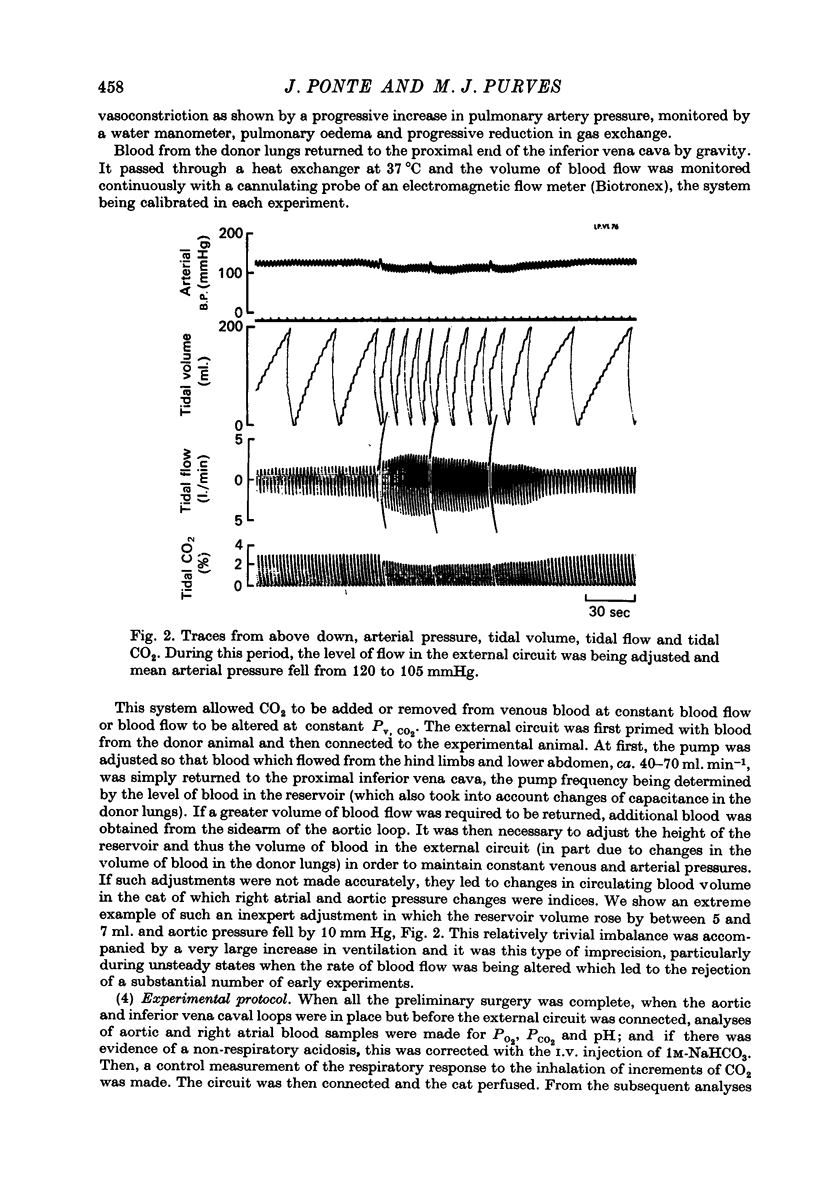

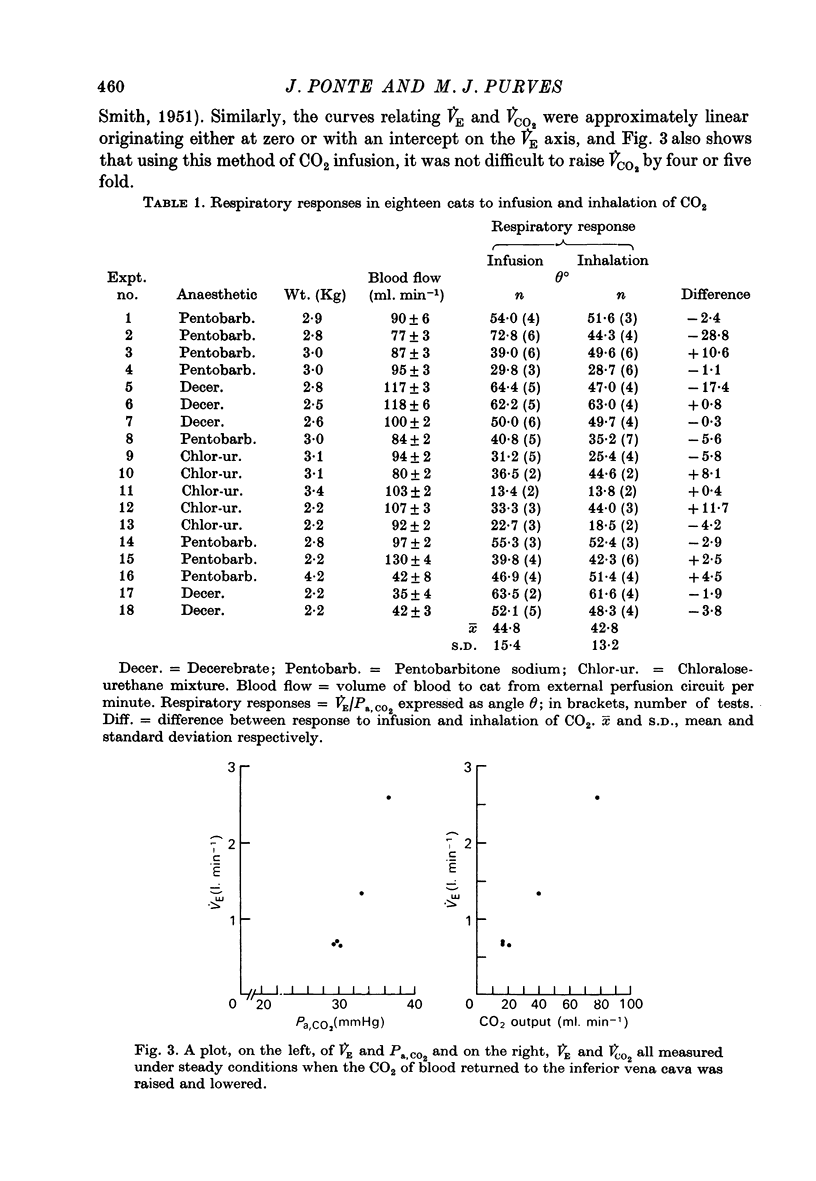
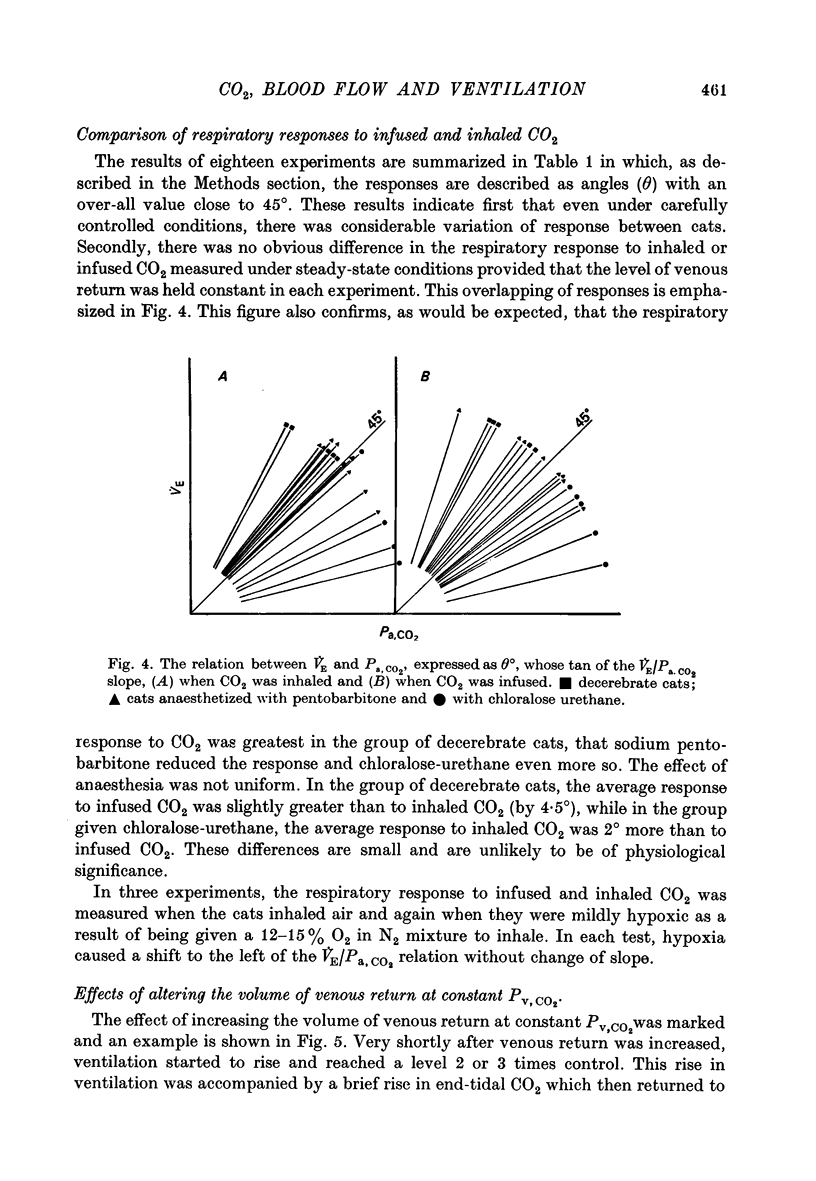






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biscoe T. J., Bradley G. W., Purves M. J. The relation between carotid body chemoreceptor discharge, carotid sinus pressure and carotid body venous flow. J Physiol. 1970 May;208(1):99–120. doi: 10.1113/jphysiol.1970.sp009108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J. Factors affecting the cat carotid chemoreceptor and cervical sympathetic activity with special reference to passive hind-limb movements. J Physiol. 1967 Jun;190(3):425–441. doi: 10.1113/jphysiol.1967.sp008219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J. Observations on carotid body chemoreceptor activity and cervical sympathetic discharge in the cat. J Physiol. 1967 Jun;190(3):413–424. doi: 10.1113/jphysiol.1967.sp008218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E., Goei J. S., Greenfield A. D., Plassaras G. C. Circulatory responses to simulated gravitational shifts of blood in man induced by exposure of the body below the iliac crests to sub-atmospheric pressure. J Physiol. 1966 Apr;183(3):607–627. doi: 10.1113/jphysiol.1966.sp007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. V., Wasserman K., Whipp B. J. Effect of beta-adrenergic blockade during exercise on ventilation and gas exchange. J Appl Physiol. 1976 Dec;41(6):886–892. doi: 10.1152/jappl.1976.41.6.886. [DOI] [PubMed] [Google Scholar]
- COLERIDGE J. C., LINDEN R. J. The effect of intravenous infusions upon the heart rate of the anaesthetized dog. J Physiol. 1955 May 27;128(2):310–319. doi: 10.1113/jphysiol.1955.sp005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROPP G. J., COMROE J. H., Jr Role of mixed venous blood PCO2 in respiratory control. J Appl Physiol. 1961 Nov;16:1029–1033. doi: 10.1152/jappl.1961.16.6.1029. [DOI] [PubMed] [Google Scholar]
- Cragg P., Patterson L., Purves M. J. The pH of brain extracellular fluid in the cat. J Physiol. 1977 Oct;272(1):137–166. doi: 10.1113/jphysiol.1977.sp012038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. M., Curtis D. R. Pharmacological studies on feline Betz cells. J Physiol. 1966 Sep;186(1):121–138. doi: 10.1113/jphysiol.1966.sp008024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Silva J. L., Gill D., Mendel D. The effects of acute haemorrhage on respiration in the cat. J Physiol. 1966 Nov;187(2):369–377. doi: 10.1113/jphysiol.1966.sp008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R. E., Crandall E. D. Time course of exchanges between red cells and extracellular fluid during CO2 uptake. J Appl Physiol. 1975 Apr;38(4):710–718. doi: 10.1152/jappl.1975.38.4.710. [DOI] [PubMed] [Google Scholar]
- GUYTON A. C., DOUGLAS B. H., LANGSTON J. B., RICHARDSON T. Q. Instantaneous increase in mean circulatory pressure and cardiac output at onset of muscular activity. Circ Res. 1962 Sep;11:431–441. doi: 10.1161/01.res.11.3.431. [DOI] [PubMed] [Google Scholar]
- HIRSCH L. J., BOYD E., KATZ L. N. EFFECT OF INTRAVENOUS VOLUME INFUSION ON HEART RATE IN UNANESTHETIZED DOGS. Am J Physiol. 1964 May;206:992–996. doi: 10.1152/ajplegacy.1964.206.5.992. [DOI] [PubMed] [Google Scholar]
- Haldane J. S., Priestley J. G. The regulation of the lung-ventilation. J Physiol. 1905 May 9;32(3-4):225–266. doi: 10.1113/jphysiol.1905.sp001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad D. D., Abboud F. M., Mark A. L., Schmid P. G. Interaction of baroreceptor and chemoreceptor reflexes. Modulation of the chemoreceptor reflex by changes in baroreceptor activity. J Clin Invest. 1974 May;53(5):1226–1236. doi: 10.1172/JCI107669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E. P., Power G. G., Longo L. D. Mathematical simulation of pulmonary O 2 and CO 2 exchange. Am J Physiol. 1973 Apr;224(4):904–917. doi: 10.1152/ajplegacy.1973.224.4.904. [DOI] [PubMed] [Google Scholar]
- KAO F. F., MICHEL C. C., MEI S. S. CARBON DIOXIDE AND PULMONARY VENTILATION IN MUSCULAR EXERCISE. J Appl Physiol. 1964 Nov;19:1075–1080. doi: 10.1152/jappl.1964.19.6.1075. [DOI] [PubMed] [Google Scholar]
- KAO F. F. Regulation of respiration during muscular activity. Am J Physiol. 1956 Apr;185(1):145–151. doi: 10.1152/ajplegacy.1956.185.1.145. [DOI] [PubMed] [Google Scholar]
- Krogh A., Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913 Oct 17;47(1-2):112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE K. D., MAYOU R. A., TORRANCE R. W. THE EFFECT OF BLOOD PRESSURE UPON CHEMORECEPTOR DISCHARGE TO HYPOXIA, AND THE MODIFICATION OF THIS EFFECT BY THE SYMPATHETIC-ADRENAL SYSTEM. Q J Exp Physiol Cogn Med Sci. 1964 Apr;49:171–183. doi: 10.1113/expphysiol.1964.sp001717. [DOI] [PubMed] [Google Scholar]
- Lamb T. W. Ventilatory responses to hind limb exercise in anesthetized cats and dogs. Respir Physiol. 1968 Dec;6(1):88–104. doi: 10.1016/0034-5687(68)90019-4. [DOI] [PubMed] [Google Scholar]
- Lamb T. W. Ventilatory responses to intravenous and inspired carbon dioxide in anesthetized cats. Respir Physiol. 1966 Dec;2(1):99–104. doi: 10.1016/0034-5687(66)90041-7. [DOI] [PubMed] [Google Scholar]
- Lewis S. M. Awake baboon's ventilatory response to venous and inhaled CO2 loading. J Appl Physiol. 1975 Sep;39(3):417–422. doi: 10.1152/jappl.1975.39.3.417. [DOI] [PubMed] [Google Scholar]
- Linton R. A., Miller R., Cameron I. R. Role of Pco2 oscillations and chemoreceptors in ventilatory response to inhaled and infused CO2. Respir Physiol. 1977 Apr;29(2):201–210. doi: 10.1016/0034-5687(77)90093-7. [DOI] [PubMed] [Google Scholar]
- Linton R. A., Miller R., Cameron I. R. Ventilatory response to CO2 inhalation and intravenous infusion of hypercapnic blood. Respir Physiol. 1976 May;26(3):383–394. doi: 10.1016/0034-5687(76)90008-6. [DOI] [PubMed] [Google Scholar]
- Lipski J., McAllen R. M., Trzebski A. Carotid baroreceptor and chemoreceptor inputs onto single medullary neurones. Brain Res. 1976 Apr 30;107(1):132–136. doi: 10.1016/0006-8993(76)90101-3. [DOI] [PubMed] [Google Scholar]
- Mills J. N. Hyperpnoea in man produced by sudden release of occluded blood. J Physiol. 1944 Sep 29;103(2):244–252. doi: 10.1113/jphysiol.1944.sp004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIELSEN M., SMITH H. Studies on the regulation of respiration in acute hypoxia; with a appendix on respiratory control during prolonged hypoxia. Acta Physiol Scand. 1952 Feb 12;24(4):293–313. doi: 10.1111/j.1748-1716.1952.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Ponte J., Purves M. J. Frequency response of carotid body chemoreceptors in the cat to changes of PaCO2, PaO2, and pHa. J Appl Physiol. 1974 Nov;37(5):635–647. doi: 10.1152/jappl.1974.37.5.635. [DOI] [PubMed] [Google Scholar]
- Ponte J., Purves M. J. Interactions of venous return and infused CO2 as stimuli to respiration in the cat [proceedings]. J Physiol. 1977 Oct;271(2):44P–44P. [PubMed] [Google Scholar]
- Ponte J., Purves M. J. Ventilation and the CO(2) content of mixed venous blood [proceedings]. J Physiol. 1976 Dec;263(1):148P–149P. [PubMed] [Google Scholar]
- Wallace R. K., Benson H., Wilson A. F. A wakeful hypometabolic physiologic state. Am J Physiol. 1971 Sep;221(3):795–799. doi: 10.1152/ajplegacy.1971.221.3.795. [DOI] [PubMed] [Google Scholar]
- Wasserman K., Whipp B. J., Casaburi R., Huntsman D. J., Castagna J., Lugliani R. Regulation of arterial PCO2 during intravenous CO2 loading. J Appl Physiol. 1975 Apr;38(4):651–656. doi: 10.1152/jappl.1975.38.4.651. [DOI] [PubMed] [Google Scholar]
- Wasserman K., Whipp B. J., Castagna J. Cardiodynamic hyperpnea: hyperpnea secondary to cardiac output increase. J Appl Physiol. 1974 Apr;36(4):457–464. doi: 10.1152/jappl.1974.36.4.457. [DOI] [PubMed] [Google Scholar]
- Wasserman K., Whipp B. J., Koyal S. N., Cleary M. G. Effect of carotid body resection on ventilatory and acid-base control during exercise. J Appl Physiol. 1975 Sep;39(3):354–358. doi: 10.1152/jappl.1975.39.3.354. [DOI] [PubMed] [Google Scholar]
- Weil J. V., Byrne-Quinn E., Sodal I. E., Kline J. S., McCullough R. E., Filley G. F. Augmentation of chemosensitivity during mild exercise in normal man. J Appl Physiol. 1972 Dec;33(6):813–819. doi: 10.1152/jappl.1972.33.6.813. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO W. S., EDWARDS M. W., Jr Homeostasis of carbon dioxide during intravenous infusion of carbon dioxide. J Appl Physiol. 1960 Sep;15:807–818. doi: 10.1152/jappl.1960.15.5.807. [DOI] [PubMed] [Google Scholar]
- Yeomans A., Porter R. R., Swank R. L. OBSERVATIONS ON CERTAIN MANIFESTATIONS OF CIRCULATORY CONGESTION PRODUCED IN DOGS BY RAPID INFUSION. J Clin Invest. 1943 Jan;22(1):33–45. doi: 10.1172/JCI101366. [DOI] [PMC free article] [PubMed] [Google Scholar]


