Abstract
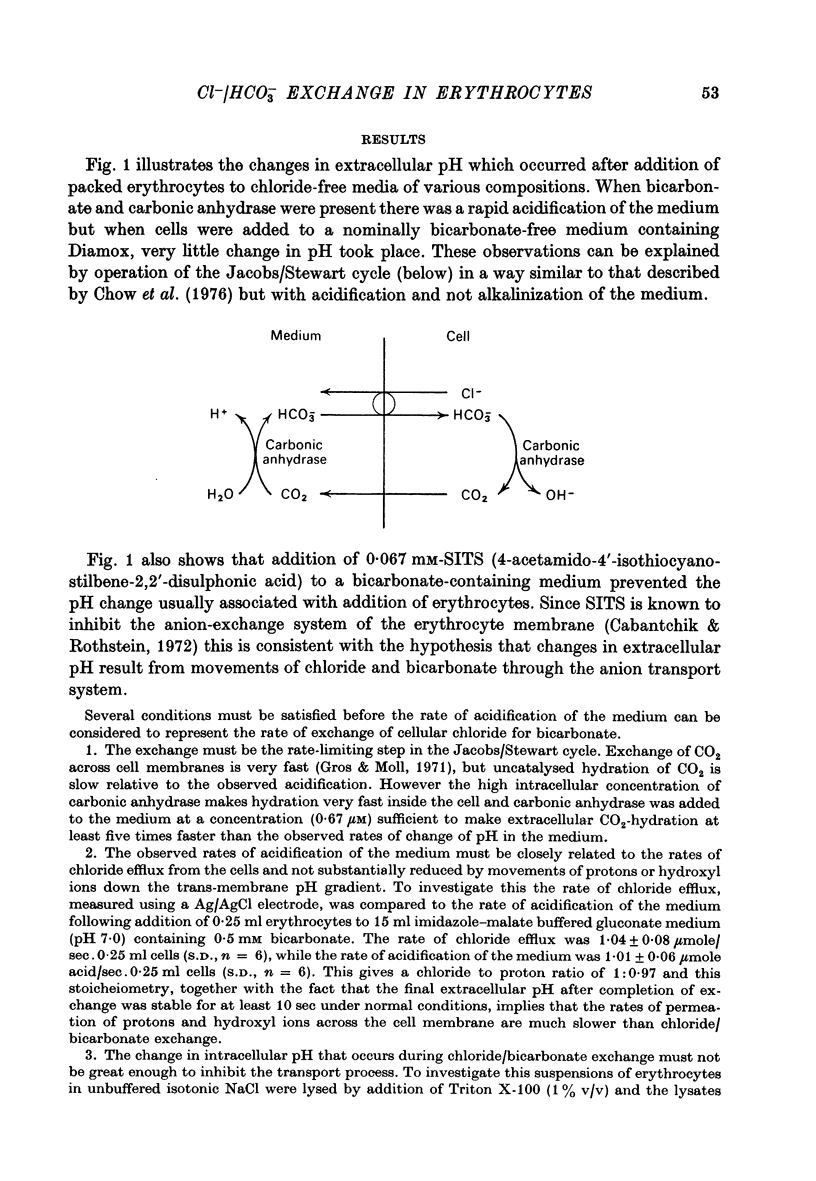
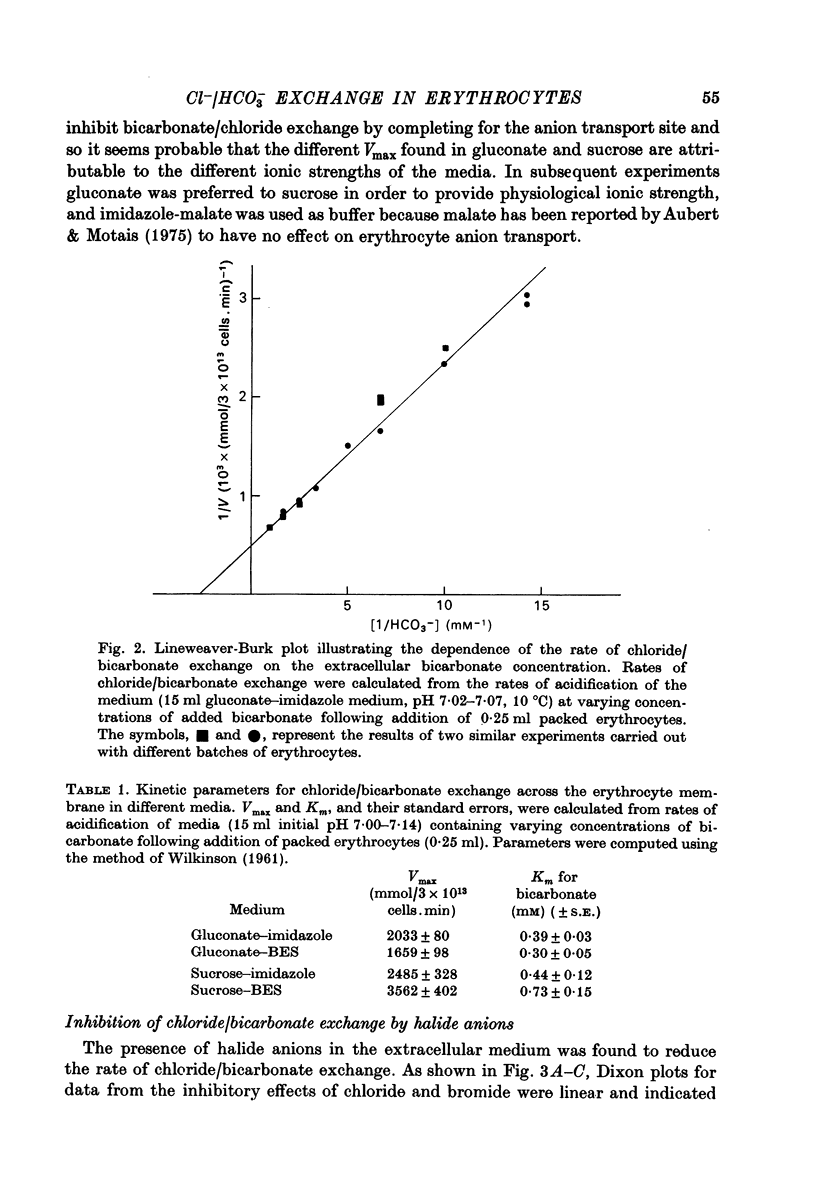
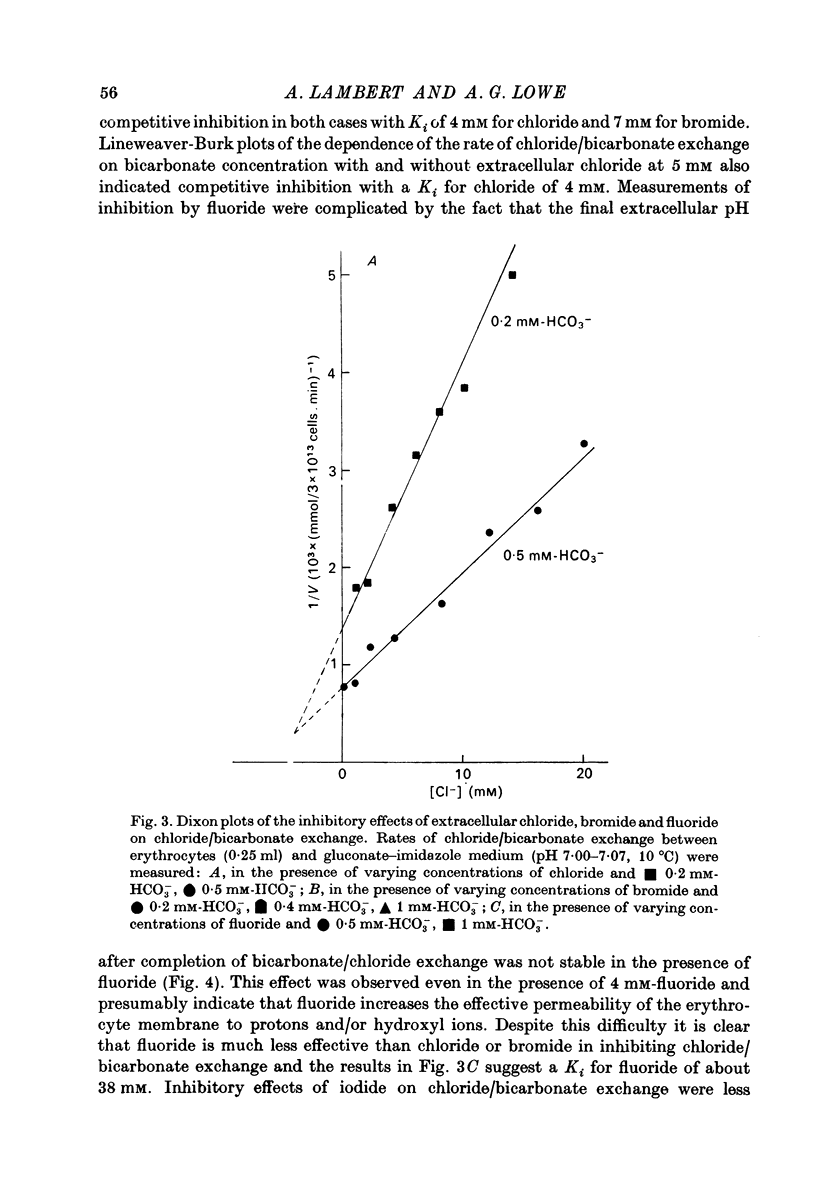
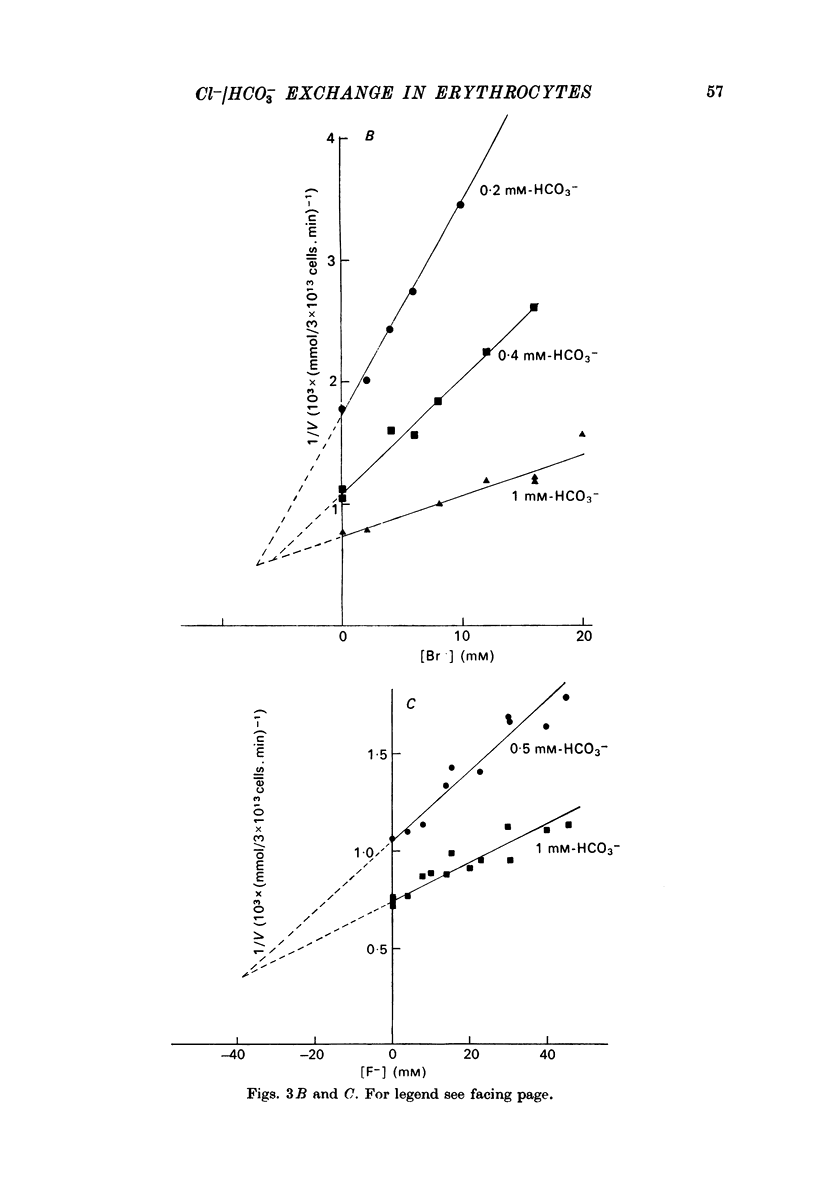
1. The exchange of chloride and bicarbonate across the human erythrocyte membrane has been followed by measuring the changes in extracellular pH which occur when chloride-rich erythrocytes are added to chloride-free media containing varying concentrations of bicarbonate and carbonic anhydrase. 2. The dependence of the rate of chloride/bicarbonate exchange on the extracellular concentration of bicarbonate was consistent with the existence of a saturable membrane anion transporter exhibiting Michaelis--Menten kinetics. In a medium containing sodium gluconate buffered to pH 7.0 with imidazole--malate the Km for bicarbonate activation of transport was 0.39 (+/- 0.03) mM and the Vmax was 2033 (+/- 80 m-mole anions exchanged/3 X 10(13) cells. min, at 10 degrees C. 3. Chloride/bicarbonate exchange was temperature-dependent with an Arrhenius activation energy of 19.4 kcal/mole in the temperature range 2--10 degrees C. 4. Exchange of intracellular chloride for extracellular bicarbonate was inhibited by the presence of extracellular halides. Inhibition by chloride, bromide and fluoride was competitive and the affinity of the transport system decreased in the order HCO-3 greater than Cl- greater than Br- greater than F-. The kinetics of inhibition by iodide were complex, but inhibitory effects of low concentrations of iodide were less than those of chloride and bromide.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubert L., Motais R. Molecular features of organic anion permeablity in ox red blood cell. J Physiol. 1975 Mar;246(1):159–179. doi: 10.1113/jphysiol.1975.sp010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Active proton transport stimulated by CO2/HCO3-, blocked by cyanide. Nature. 1976 Jan 22;259(5540):240–241. doi: 10.1038/259240a0. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Chow E. I., Crandall E. D., Forster R. E. Kinetics of bicarbonate-chloride exchange across the human red blood cell membrane. J Gen Physiol. 1976 Dec;68(6):633–652. doi: 10.1085/jgp.68.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin J. L., Motais R., Sola F. Transmembrane exchange of chloride with bicarbonate ion in mammalian red blood cells: evidence for a sulphonamide-sensitive "carrier". J Physiol. 1975 Dec;253(2):385–399. doi: 10.1113/jphysiol.1975.sp011195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin J. L., Motais R. The role of carbonic anhydrase inhibitors on anion permeability into ox red blood cells. J Physiol. 1976 Mar;256(1):61–80. doi: 10.1113/jphysiol.1976.sp011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C. A spectrophotometric method for the simultaneous determination of myoglobin and hemoglobin in extracts of human muscle. Acta Chem Scand. 1948;2(3):264–289. doi: 10.3891/acta.chem.scand.02-0264. [DOI] [PubMed] [Google Scholar]
- Dalmark M. Chloride transport in human red cells. J Physiol. 1975 Aug;250(1):39–64. doi: 10.1113/jphysiol.1975.sp011042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmark M. Effects of halides and bicarbonate on chloride transport in human red blood cells. J Gen Physiol. 1976 Feb;67(2):223–234. doi: 10.1085/jgp.67.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmark M., Wieth J. O. Temperature dependence of chloride, bromide, iodide, thiocyanate and salicylate transport in human red cells. J Physiol. 1972 Aug;224(3):583–610. doi: 10.1113/jphysiol.1972.sp009914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirken M. N., Mook H. W. The rate of gas exchange between blood cells and serum. J Physiol. 1931 Dec 17;73(4):349–360. doi: 10.1113/jphysiol.1931.sp002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Trapping of sodium, potassium, sucrose, and albumin in the packed cell column of the hematocrit. Acta Physiol Scand. 1967 Sep;71(1):105–112. doi: 10.1111/j.1748-1716.1967.tb03715.x. [DOI] [PubMed] [Google Scholar]
- Gros G., Moll W. The diffusion of carbon dioxide in erythrocytes and hemoglobin solutions. Pflugers Arch. 1971;324(3):249–266. doi: 10.1007/BF00586422. [DOI] [PubMed] [Google Scholar]
- Gunn R. B., Dalmark M., Tosteson D. C., Wieth J. O. Characteristics of chloride transport in human red blood cells. J Gen Physiol. 1973 Feb;61(2):185–206. doi: 10.1085/jgp.61.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H., Scorah K., Fasold H., Passow H. Sidedness of the inhibitory action of disulfonic acids on chloride equilibrium exchange and net transport across the human erythrocyte membrane. FEBS Lett. 1976 Feb 15;62(2):182–185. doi: 10.1016/0014-5793(76)80048-8. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Boron W. F. Role of choloride transport in regulation of intracellular pH. Nature. 1976 Nov 4;264(5581):73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


