Abstract
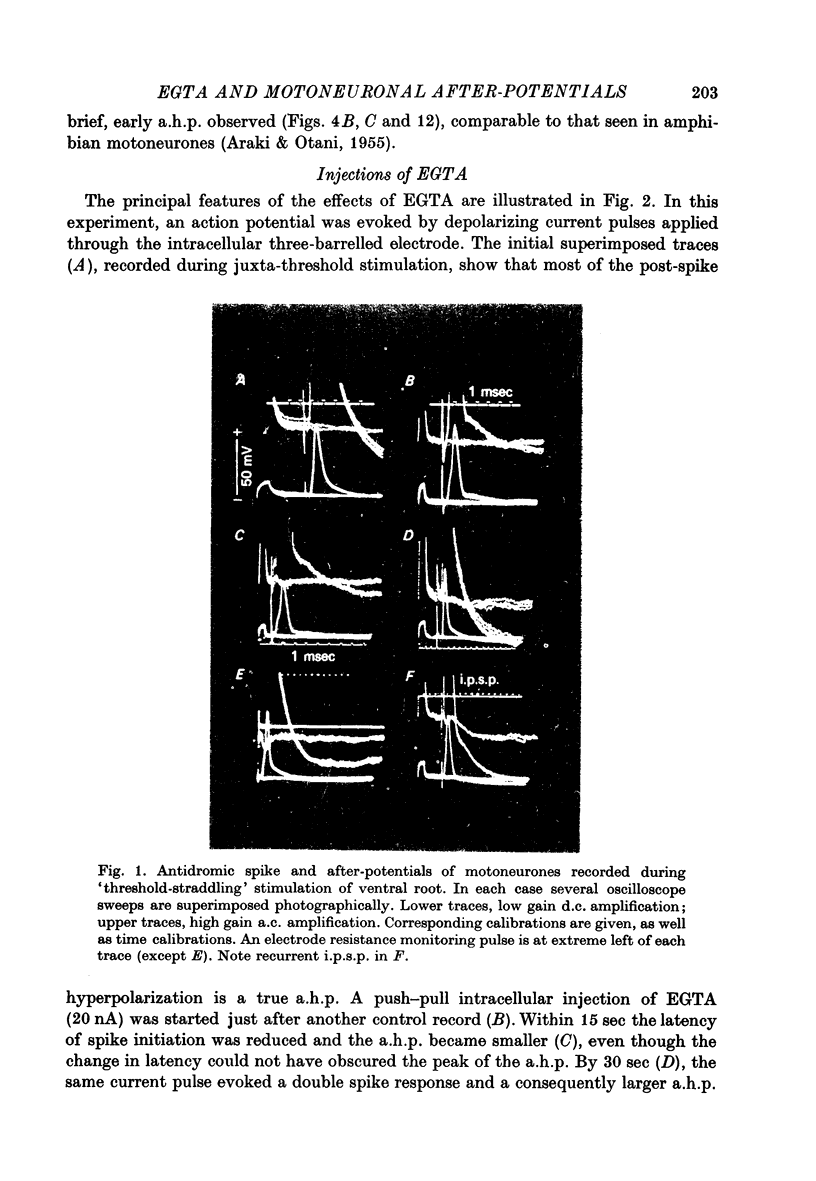
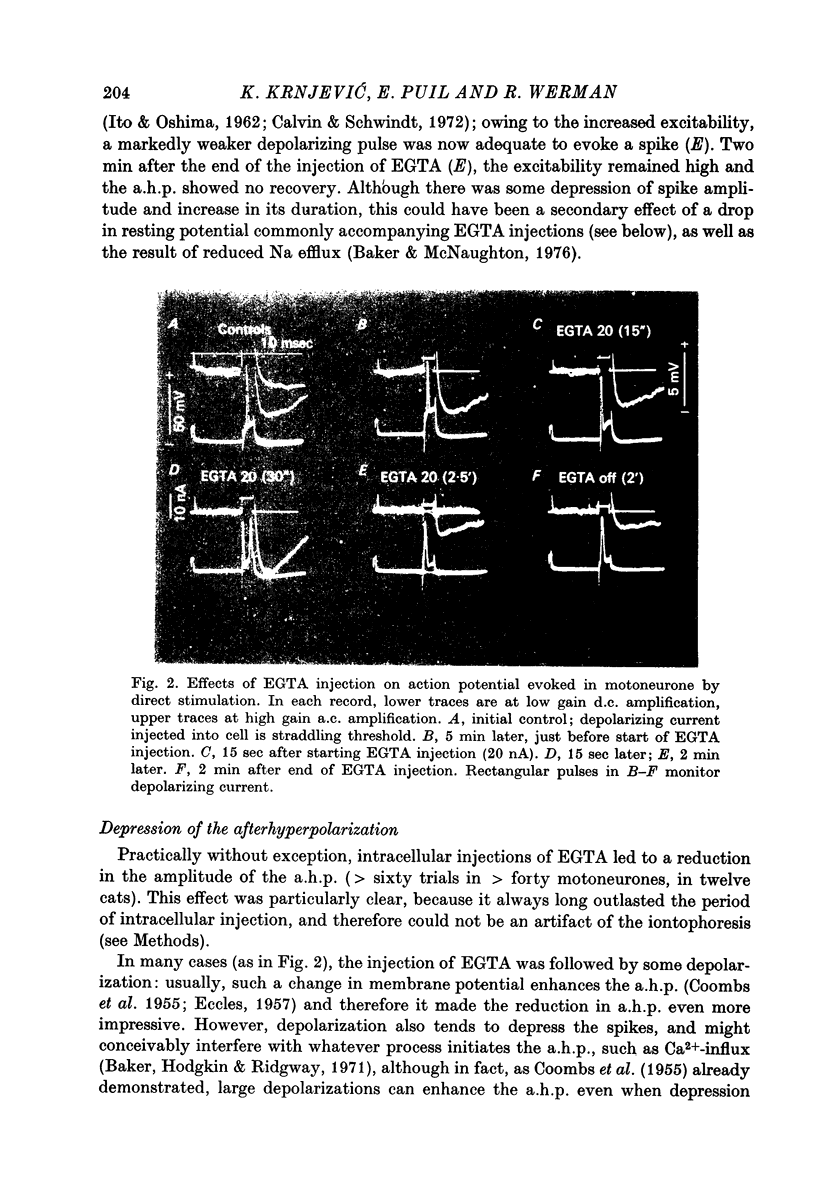
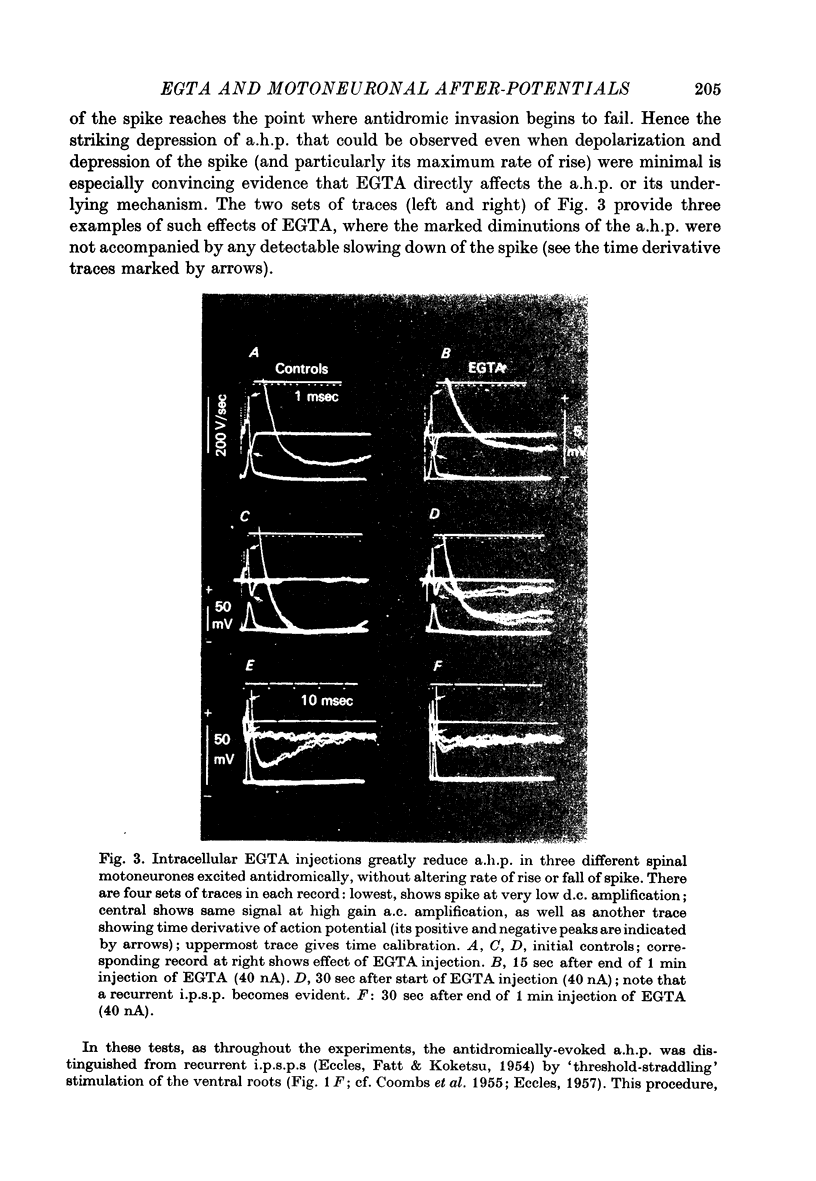
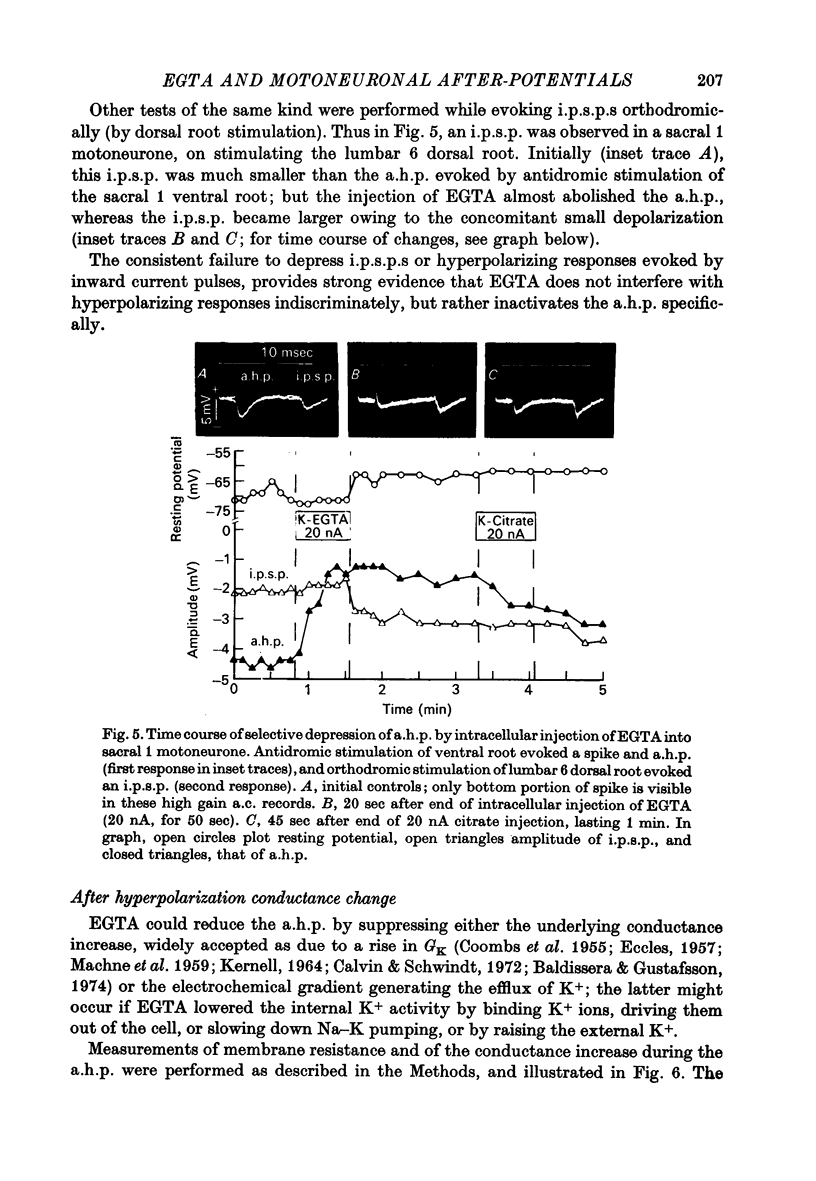
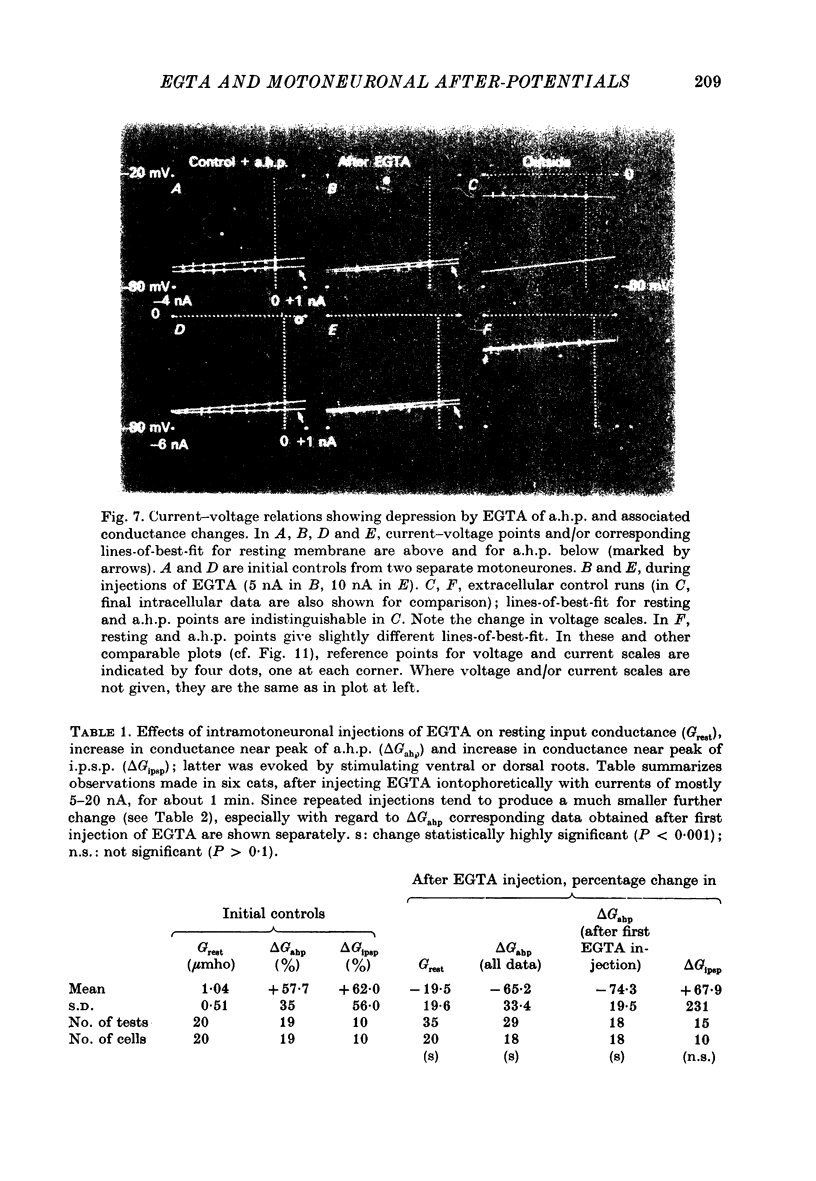
1. Intracellular iontophoretic injections of EGTA (5--20 nA) into cat spinal motoneurones consistently greatly reduce the amplitude of the delayed after hyperpolarization (a.h.p.) that follows the spike. 2. This effect is accompanied by a large reduction (on average by 3/4) in the marked increase in input conductance normally associated with the a.h.p. 3. There is also a consistent, though less regular, tendency for the resting input conductance to decrease (on average by 1/5), as well as some depolarization. 4. Recovery of the a.h.p., the associated conductance increase and the resting conductance is ver slow. It is sometimes accelerated by injections of citrate and Cl-, or CA2+. 5. Other hyperpolarizing phenomena, such as recurrent or othodromically-evoked i.p.s.p.s, are not depressed by injections of EGTA. 6. When depolarization is minimal EGTA injections that markedly depress the a.h.p. do not affect the rate of rise or fall of the spike. If, as a result of depolarization, an early a.h.p. is visible, it is patently insensitive to EGTA. 7. The post-spike depolarizing after-potential (delayed depolarization) is not obviously affected by EGTA, apart from the usual diminution seen during depolarization. 8. Since the main action of EGTA is to bind free Ca2+, the marked depression of the a.h.p. indicates that the sharp increase in K conductance which generates the a.h.p. is probably caused by a influx of Ca2+ accompanying the action potential. It is suggested that this inward Ca2+ current may be manifested in the depolarizing after-potential.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., OTANI T. Response of single motoneurons to direct stimulation in toad's spinal cord. J Neurophysiol. 1955 Sep;18(5):472–485. doi: 10.1152/jn.1955.18.5.472. [DOI] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952 Aug;117(4):431–460. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
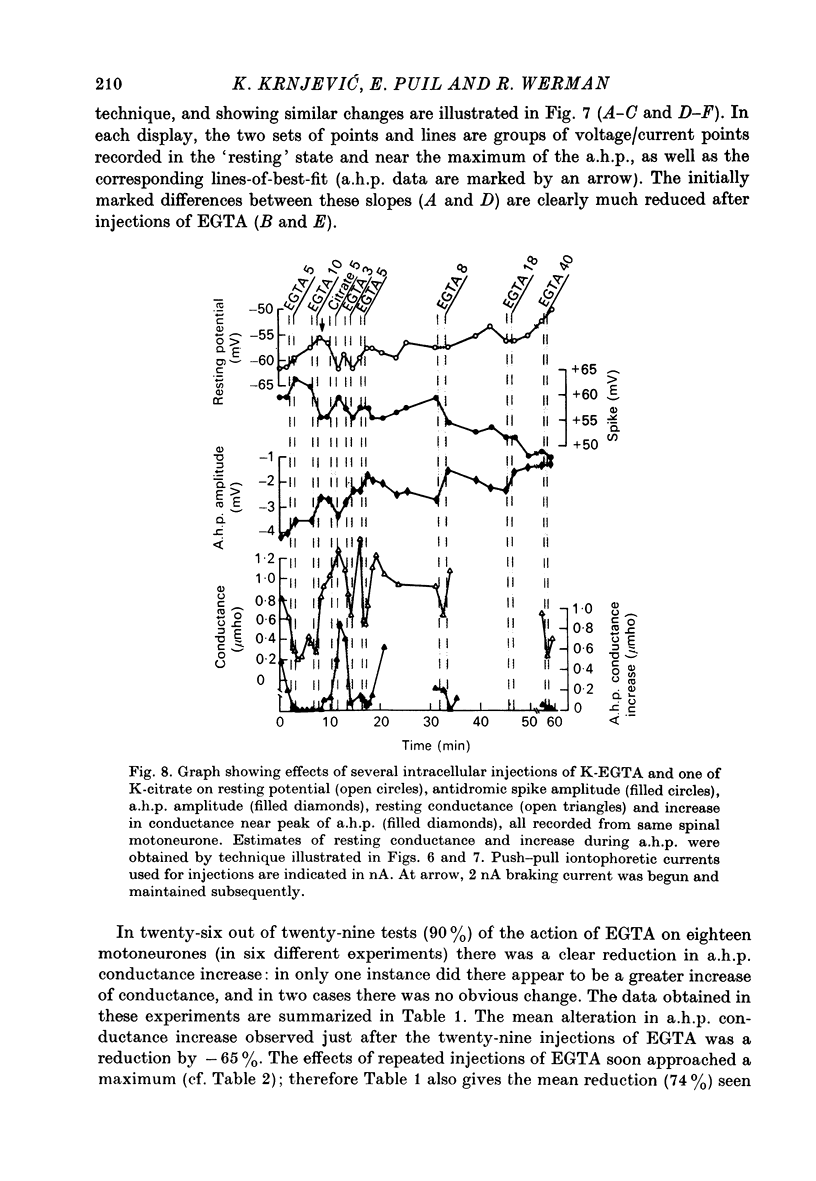
- BROOKS C. M., DOWNMAN C. B. B., ECCLES J. C. After-potentials and excitability of spinal motoneurones following antidromic activation. J Neurophysiol. 1950 Jan;13(1):9–38. doi: 10.1152/jn.1950.13.1.9. [DOI] [PubMed] [Google Scholar]
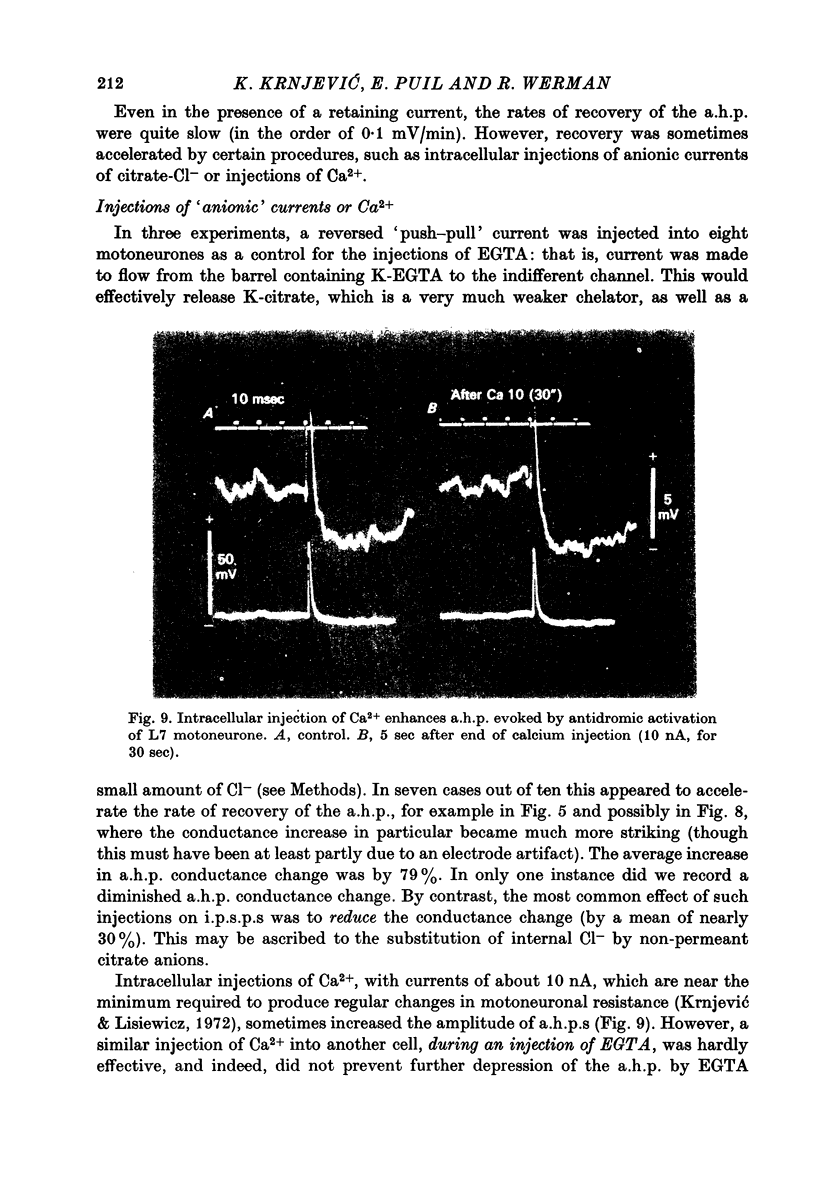
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Schlaepfer W. Proceedings: Calcium uptake by axoplasm extruded from giant axons of Loligo. J Physiol. 1975 Jul;249(1):37P–38P. [PubMed] [Google Scholar]
- Baldissera F., Gustafsson B. Afterhyperpolarization conductance time course in lumbar motoneurones of the cat. Acta Physiol Scand. 1974 Aug;91(4):512–527. doi: 10.1111/j.1748-1716.1974.tb05707.x. [DOI] [PubMed] [Google Scholar]
- Baldissera F. Relationships between the spike components and the delayed depolarization in cat spinal neurones. J Physiol. 1976 Jul;259(2):325–338. doi: 10.1113/jphysiol.1976.sp011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron D. H., Matthews B. H. The interpretation of potential changes in the spinal cord. J Physiol. 1938 Apr 14;92(3):276–321. doi: 10.1113/jphysiol.1938.sp003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassingthwaighte J. B., Fry C. H., McGuigan J. A. Relationship between internal calcium and outward current in mammalian ventricular muscle; a mechanism for the control of the action potential duration? J Physiol. 1976 Oct;262(1):15–37. doi: 10.1113/jphysiol.1976.sp011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T., Lynch C. Effects of internal divalent cations on voltage-clamped squid axons. J Gen Physiol. 1974 Jun;63(6):675–689. doi: 10.1085/jgp.63.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Brown H. M. Light response of a giant Aplysia neuron. J Gen Physiol. 1973 Sep;62(3):239–254. doi: 10.1085/jgp.62.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The electrical properties of the motoneurone membrane. J Physiol. 1955 Nov 28;130(2):291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin W. H., Schwindt P. C. Steps in production of motoneuron spikes during rhythmic firing. J Neurophysiol. 1972 May;35(3):297–310. doi: 10.1152/jn.1972.35.3.297. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin W. T., Bennett M. V. Calcium-activated conductance in skate electroreceptors: voltage clamp experiments. J Gen Physiol. 1977 Feb;69(2):145–182. doi: 10.1085/jgp.69.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambach G. E., Erulkar S. D. The action of calcium at spinal neurones of the frog. J Physiol. 1973 Feb;228(3):799–817. doi: 10.1113/jphysiol.1973.sp010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink R., Lüttgau H. C. An evaluation of the membrane constants and the potassium conductance in metabolically exhausted muscle fibres. J Physiol. 1976 Dec;263(2):215–238. doi: 10.1113/jphysiol.1976.sp011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., KERNELL D., SMITH R. S. DELAYED DEPOLARIZATION AND THE REPETITIVE RESPONSE TO INTRACELLULAR STIMULATION OF MAMMALIAN MOTONEURONES. J Physiol. 1963 Oct;168:890–910. doi: 10.1113/jphysiol.1963.sp007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Control of the delayed outward potassium currents in bursting pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):349–382. doi: 10.1113/jphysiol.1976.sp011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERNELL D. THE DELAYED DEPOLARIZATION IN CAT AND RAT MOTONEURONES. Prog Brain Res. 1964;12:42–55. doi: 10.1016/s0079-6123(08)60616-0. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Lisiewicz A. Injections of calcium ions into spinal motoneurones. J Physiol. 1972 Sep;225(2):363–390. doi: 10.1113/jphysiol.1972.sp009945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Puil E., VanMeter W. G., Werman R. Activation and inactivation of K channels in cat spinal motorneurones [proceedings]. J Physiol. 1976 Dec;263(1):126P–127P. [PubMed] [Google Scholar]
- Krnjević K., Puil E., Werman R. Intracellular Mg2+ increases neuronal excitability. Can J Physiol Pharmacol. 1976 Feb;54(1):73–77. doi: 10.1139/y76-012. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Puil E., Werman R. Is cyclic guanosine monophosphate the internal 'second messenger' for cholinergic actions on central neurons? Can J Physiol Pharmacol. 1976 Apr;54(2):172–176. doi: 10.1139/y76-029. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Puil E., Werman R. Significance of 2,4-dinitrophenol action on spinal motoneurones. J Physiol. 1978 Feb;275:225–239. doi: 10.1113/jphysiol.1978.sp012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. Effects of Ba2+ and tetraethylammonium on cortical neurones. J Physiol. 1971 May;215(1):223–245. doi: 10.1113/jphysiol.1971.sp009466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevíc K., Puil E., Werman R. Evidence for Ca2+-activated K+ conductance in cat spinal motoneurons from intracellular EGTA injections. Can J Physiol Pharmacol. 1975 Dec;53(6):1214–1218. doi: 10.1139/y75-171. [DOI] [PubMed] [Google Scholar]
- LLOYD D. P. C. After-currents, after-potentials, excitability, and ventral root electrotonus in spinal motoneurons. J Gen Physiol. 1951 Nov;35(2):289–321. doi: 10.1085/jgp.35.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew V. L., Ferreira H. G. Variable Ca sensitivity of a K-selective channel in intact red-cell membranes. Nature. 1976 Sep 23;263(5575):336–338. doi: 10.1038/263336a0. [DOI] [PubMed] [Google Scholar]
- Logan J. G., O'Donvan D. J. Some characteristics of the noradrenaline activated ATPases of cerebral synaptic membranes [proceedings]. J Physiol. 1976 Dec;263(2):246P–247P. [PubMed] [Google Scholar]
- MACHNE X., FADIGA E., BROOKHART J. M. Antidromic and synaptic activation of frog motor neurons. J Neurophysiol. 1959 Sep;22:483–503. doi: 10.1152/jn.1959.22.5.483. [DOI] [PubMed] [Google Scholar]
- Magherini P. C., Precht W. Electrical properties of frog motoneurons in the in situ spinal cord. J Neurophysiol. 1976 May;39(3):459–473. doi: 10.1152/jn.1976.39.3.459. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol. 1977 Mar;265(3):867–879. doi: 10.1113/jphysiol.1977.sp011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minota S. Calcium ions and the post-tetanic hyperpolarization of bullfrog sympathetic ganglion cells. Jpn J Physiol. 1974 Oct;24(5):501–512. doi: 10.2170/jjphysiol.24.501. [DOI] [PubMed] [Google Scholar]
- Mounier Y., Vassort G. Evidence for a transient potassium membrane current dependent on calcium influx in crab muscle fibre. J Physiol. 1975 Oct;251(3):609–625. doi: 10.1113/jphysiol.1975.sp011111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Burke R. E. Delayed depolarization in cat spinal motoneurons. Exp Neurol. 1967 Jan;17(1):16–26. doi: 10.1016/0014-4886(67)90118-5. [DOI] [PubMed] [Google Scholar]
- Nishi S., North R. A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973 Jun;231(3):471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Parnas I., Strumwasser F. Mechanisms of long-lasting inhibition of a bursting pacemaker neuron. J Neurophysiol. 1974 Jul;37(4):609–620. doi: 10.1152/jn.1974.37.4.609. [DOI] [PubMed] [Google Scholar]
- Rojas E., Taylor R. E. Simultaneous measurements of magnesium, calcium and sodium influxes in perfused squid giant axons under membrane potential control. J Physiol. 1975 Oct;252(1):1–27. doi: 10.1113/jphysiol.1975.sp011131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Calcium ion distribution in cytoplasm visualised by aequorin: diffusion in cytosol restricted by energized sequestering. Science. 1975 Dec 19;190(4220):1204–1206. doi: 10.1126/science.1198106. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Kurchavyi G. G. Effects of trans-membrane polarization and TEA injection on monosynaptic actions from motor cortex, red nucleus and group Ia afferents on lumbar motoneurons in the monkey. Brain Res. 1974 Dec 20;82(1):49–67. doi: 10.1016/0006-8993(74)90892-0. [DOI] [PubMed] [Google Scholar]
- Vassort G. Voltage-clamp analysis of transmembrane ionic currents in guinea-pig myometrium: evidence for an initial potassium activation triggered by calcium influx. J Physiol. 1975 Nov;252(3):713–734. doi: 10.1113/jphysiol.1975.sp011167. [DOI] [PMC free article] [PubMed] [Google Scholar]


