Abstract
1. Low levels of insuling stimulate transendothelial fluid transport from preswollen stroma to aqueous in rabbit corneal preparations. The rate of stromal thinning at the end of the first hour averages 30% faster with insulin, 3.5 x 10(-22) M (4.8 micromicron/ml.), than that of the paired control. This concentration is about the physiological level in rabbit aqueous. 2. The stimulation with insulin is transient. Rates of thinning average higher but not significantly different from control rates by the second hour. 3. High levels of insulin between 3.5 x 10(-9) M (480 micromicron/ml.) and 2.0 x 10(-6) M (2.75 X 10(5) micromicron/ml.) inhibit fluid transport. The inhibition at the low end of this range of concentrations becomes more pronounced with longer perfusion times but appears not to exceed ca. 50% of the control rate. 4. Ouabain also induces a biphasic effect on fluid transport which is characteristically different from that with insulin. The maximal stimulation observed at all times occurred with a fixed concentration of 10(-10) M. The stimulation is not transient but increases throughout the duration of the perfusion; the average rate is elevated 50% above the control rate by the third hour. 5. The transition from a stimulatory to an inhibitory effect occurs consistently at ca. 10(-8) M with ouabain, while a similar transition with insulin occurs at ca. 10(-9) M and appears to shift towards slightly higher concentrations during a 3 hr perfusion period. 6. Inhibition of fluid transport with ouabain, 3 x 10(-7) M, is increased from ca. 50% after 1 hr to more than 70% at the end of the third hour of perfusion. 7. The combined presence of stimulatory concentrations of ouabain and insulin affects tromal thinning in a manner resembling the effect of ouabain alone more than that of insulin; additive effects could not be discriminated. Progressively raising the concentration of insulin to a level (10(-8) M) that alone inhibits stromal thinning, ultimately abolishes the stimulatory effect of ouabain. Based on other evidence and current models of drug/hormone-membrane interaction, these results can be interpreted to indicate a concentration-dependent interaction between receptor complexes of ouabain and insulin with (Na+ + K+)-ATPase.
Full text
PDF


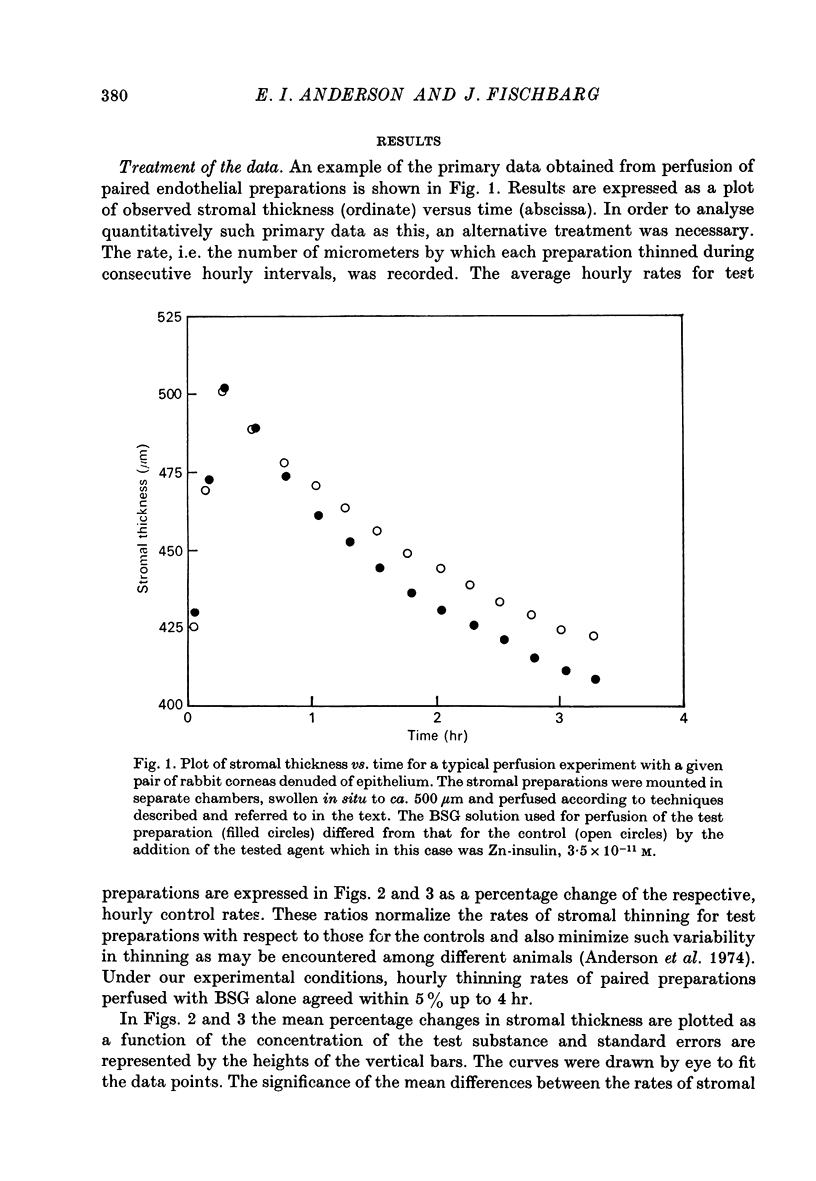

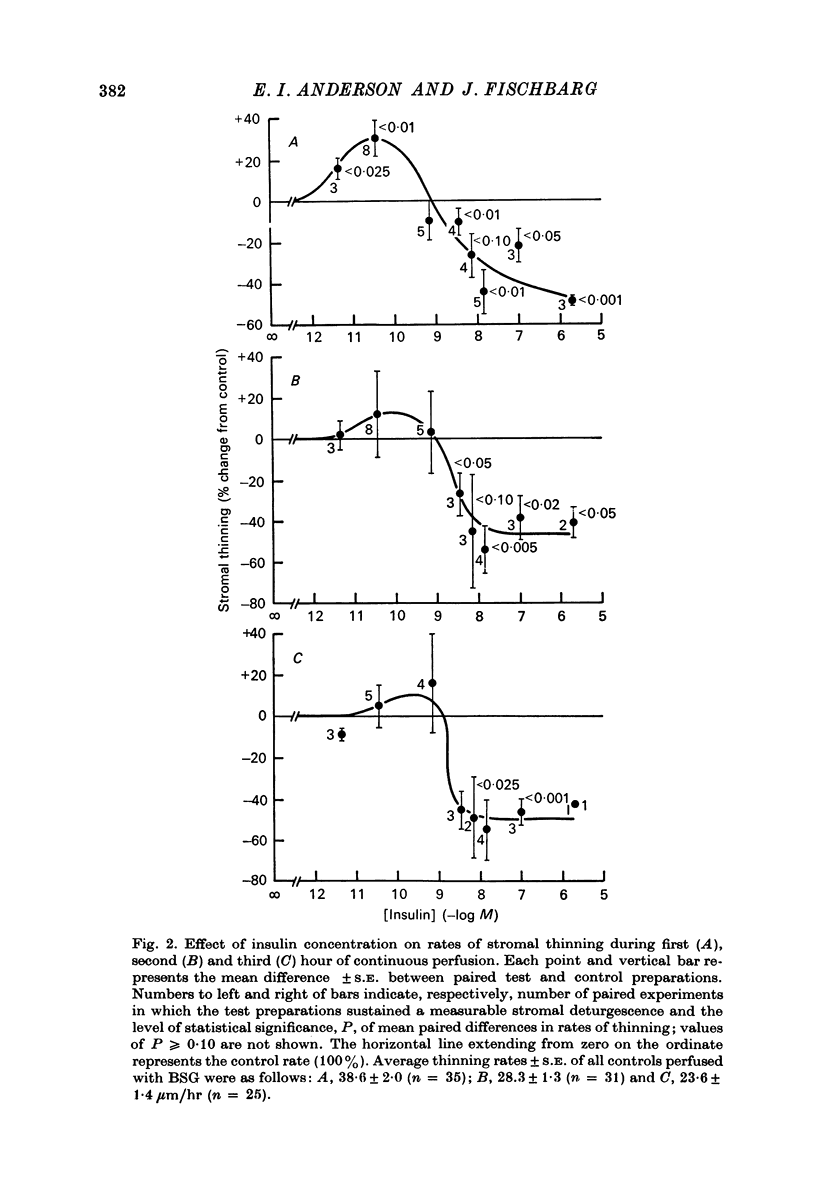
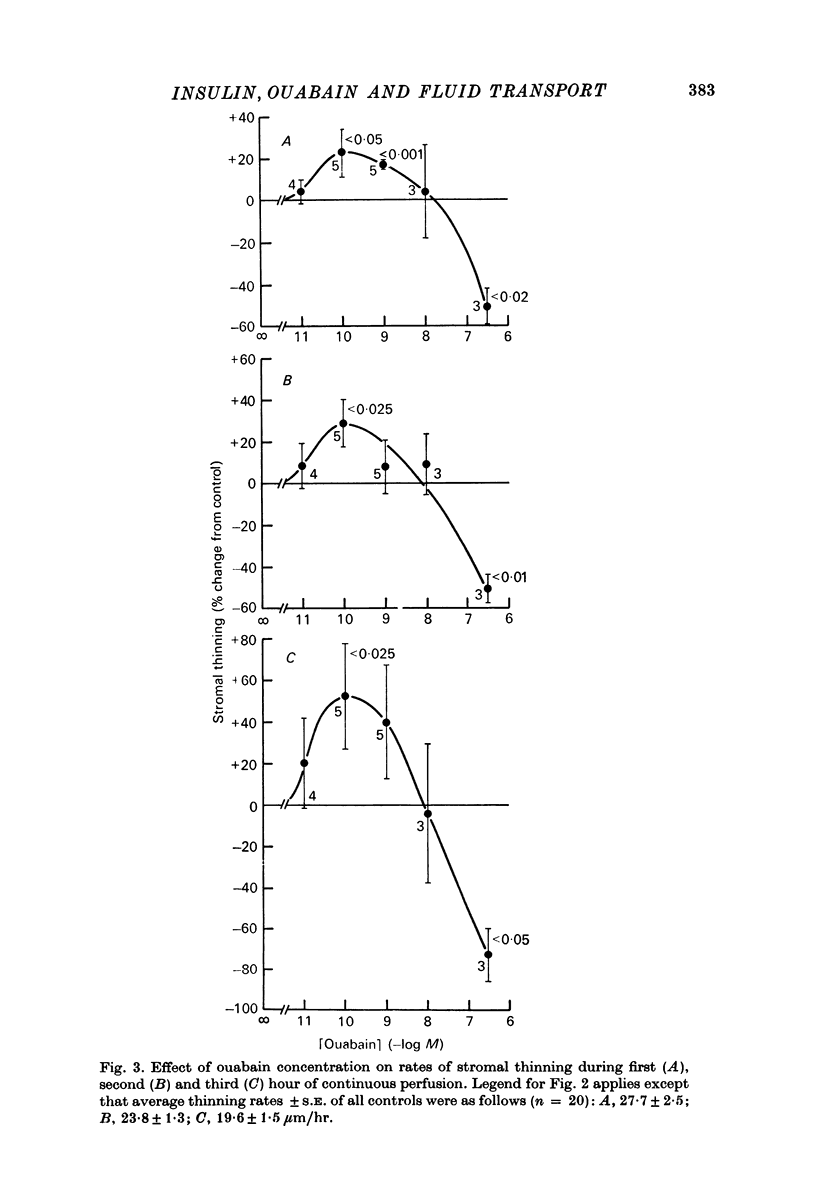
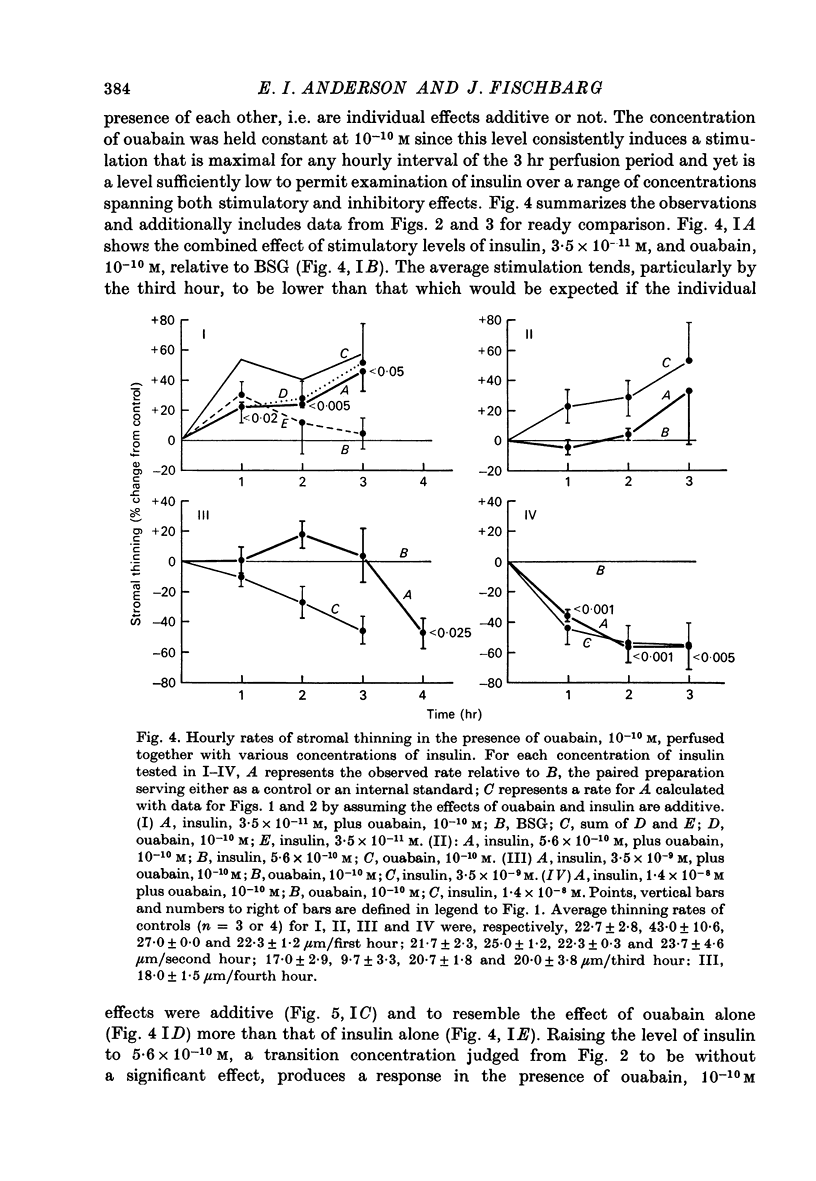





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. I., Fischbarg J., Spector A. Disulfide stimulation of fluid transport and effect on ATP level in rabbit corneal endothelium. Exp Eye Res. 1974 Jul;19(1):1–10. doi: 10.1016/0014-4835(74)90066-9. [DOI] [PubMed] [Google Scholar]
- Anderson E. I., Fischbarg J., Spector A. Fluid transport, ATP level and ATPase activities in isolated rabbit corneal endothelium. Biochim Biophys Acta. 1973 May 25;307(3):557–562. doi: 10.1016/0005-2736(73)90300-3. [DOI] [PubMed] [Google Scholar]
- Blatt L. M., McVerry P. H., Kim K. H. Regulation of hepatic glycogen synthetase of Rana catesbeiana. Inhibition of the action of insulin by ouabain. J Biol Chem. 1972 Oct 25;247(20):6551–6554. [PubMed] [Google Scholar]
- Cahill G. F., Jr The Banting Memorial Lecture 1971. Physiology of insulin in man. Diabetes. 1971 Dec;20(12):785–799. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- Creese R. Sodium fluxes in diaphragm muscle and the effects of insulin and serum proteins. J Physiol. 1968 Jul;197(2):255–278. doi: 10.1113/jphysiol.1968.sp008558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Dahl J. L., Hokin L. E. The sodium-potassium adenosinetriphosphatase. Annu Rev Biochem. 1974;43(0):327–356. doi: 10.1146/annurev.bi.43.070174.001551. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Henderson J. R. Insulin in bile and other body fluids. Lancet. 1967 Jun 10;1(7502):1256–1257. doi: 10.1016/s0140-6736(67)92718-3. [DOI] [PubMed] [Google Scholar]
- Dikstein S., Maurice D. M. The metabolic basis to the fluid pump in the cornea. J Physiol. 1972 Feb;221(1):29–41. doi: 10.1113/jphysiol.1972.sp009736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J. Active and passive properties of the rabbit corneal endothelium. Exp Eye Res. 1973 May 10;15(5):615–638. doi: 10.1016/0014-4835(73)90071-7. [DOI] [PubMed] [Google Scholar]
- Fischbarg J., Lim J. J. Role of cations, anions and carbonic anhydrase in fluid transport across rabbit corneal endothelium. J Physiol. 1974 Sep;241(3):647–675. doi: 10.1113/jphysiol.1974.sp010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavryck W. A., Moore R. D., Thompson R. C. Effect of insulin upon membrane-bound (Na+ + K+)-ATPase extracted from frog skeletal muscle. J Physiol. 1975 Oct;252(1):43–58. doi: 10.1113/jphysiol.1975.sp011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenson L. E., Okigaki T., Andersson M., Molson J., Davidson M. B. Fine structural and growth characteristics of cultured rat liver cells. Insulin effects. Exp Cell Res. 1972 Mar;71(1):49–58. doi: 10.1016/0014-4827(72)90262-5. [DOI] [PubMed] [Google Scholar]
- Greco A. V., Ghirlanda G., Fedeli G., Fenici R., Bertoni G., Gambassi G. Immunoassay of insulin in the aqueous humour of diabetics and non-diabetics with or without cataract. J Med. 1973;4(4):197–201. [PubMed] [Google Scholar]
- Grinstein S., Erlij D. Insulin unmasks latent sodium pump sites in frog muscle. Nature. 1974 Sep 6;251(5470):57–58. doi: 10.1038/251057a0. [DOI] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Wilson E. E., Good R. A., Coffey R. G. Direct action of insulin on plasma membrane ATPase activity in human lymphocytes. Nat New Biol. 1972 Feb 9;235(58):174–177. doi: 10.1038/newbio235174a0. [DOI] [PubMed] [Google Scholar]
- Ho R. J., Jeanrenaud B. Insulin-like action of ouabain. I. Effect on carbohydrate metabolism. Biochim Biophys Acta. 1967 Aug 8;144(1):61–73. doi: 10.1016/0005-2760(67)90077-x. [DOI] [PubMed] [Google Scholar]
- Ho R. J., Jeanrenaud B., Posternak T., Renold A. E. Insulin-like action of ouabain. II. Primary antilipolytic effect through inhibition of adenyl cyclase. Biochim Biophys Acta. 1967 Aug 8;144(1):74–82. doi: 10.1016/0005-2760(67)90078-1. [DOI] [PubMed] [Google Scholar]
- Hodson S. Evidence for a bicarbonate-dependent sodium pump in corneal endothelium. Exp Eye Res. 1971 Jan;11(1):20–29. doi: 10.1016/s0014-4835(71)80060-x. [DOI] [PubMed] [Google Scholar]
- KERNAN R. P. The role of lactate in the active excretion of sodium by frog muscle. J Physiol. 1962 Jun;162:129–137. doi: 10.1113/jphysiol.1962.sp006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESTENS P. J., HAXHE J. J., LAMBOTTE L., LAMBOTTE C. THE EFFECT OF INSULIN ON THE UPTAKE OF POTASSIUM AND PHOSPHATE BY THE ISOLATED PERFUSED CANINE LIVER. Metabolism. 1963 Oct;12:941–950. [PubMed] [Google Scholar]
- Lee K. S., Klaus W. The subcellular basis for the mechanism of inotropic action of cardiac glycosides. Pharmacol Rev. 1971 Sep;23(3):193–261. [PubMed] [Google Scholar]
- Legros F., Saines M., Conard V. Competitive effect of insulin and ouabain on metabolism of Acetabularia mediterranea. Arch Int Physiol Biochim. 1973 Oct;81(4):745–754. doi: 10.3109/13813457309074480. [DOI] [PubMed] [Google Scholar]
- Lord R. S., Gubensek F., Rupley J. A. Insulin self-association. Spectrum changes and thermodynamics. Biochemistry. 1973 Oct 23;12(22):4385–4391. doi: 10.1021/bi00746a014. [DOI] [PubMed] [Google Scholar]
- Lostroh A. J., Krahl M. E. Insulin action. Accumulation in vitro of Mg 2 + and K + in rat uterus: ion pump activity. Biochim Biophys Acta. 1973 Jan 2;291(1):260–268. doi: 10.1016/0005-2736(73)90417-3. [DOI] [PubMed] [Google Scholar]
- MACROBBIE E. A., USSING H. H. Osmotic behaviour of the epithelial cells of frog skin. Acta Physiol Scand. 1961 Nov-Dec;53:348–365. doi: 10.1111/j.1748-1716.1961.tb02293.x. [DOI] [PubMed] [Google Scholar]
- MCCLANE T. K. A BIPHASIC ACTION OF OUABAIN ON SODIUM TRANSPORT IN THE TOAD BLADDER. J Pharmacol Exp Ther. 1965 Apr;148:106–110. [PubMed] [Google Scholar]
- Maurice D. M. The location of the fluid pump in the cornea. J Physiol. 1972 Feb;221(1):43–54. doi: 10.1113/jphysiol.1972.sp009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. D. Effect of insulin upon the sodium pump in frog skeletal muscle. J Physiol. 1973 Jul;232(1):23–45. doi: 10.1113/jphysiol.1973.sp010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. F., Lasseter K. C., Melvin S. L. Stimulation of Na+ and K+ dependent adenosine triphosphatase by ouabain. Arch Biochem Biophys. 1966 Mar;113(3):629–633. doi: 10.1016/0003-9861(66)90240-2. [DOI] [PubMed] [Google Scholar]
- Rogers K. T. Levels of (Na+ + K+)-activated and Mg2+-activated ATPase activity in bovine and feline corneal endothelium and epithelium. Biochim Biophys Acta. 1968 Aug;163(1):50–56. doi: 10.1016/0005-2736(68)90032-1. [DOI] [PubMed] [Google Scholar]
- Sperling R., Steinberg I. Z. Simultaneous reduction and mercuration of disulfide bond A6-A11 of insulin by monovalent mercury. Biochemistry. 1974 May 7;13(10):2007–2013. doi: 10.1021/bi00707a002. [DOI] [PubMed] [Google Scholar]
- Trenberth S. M., Mishima S. The effect of ouabain on the rabbit corneal endothelium. Invest Ophthalmol. 1968 Feb;7(1):44–52. [PubMed] [Google Scholar]
- ZIERLER K. L., RABINOWITZ D. EFFECT OF VERY SMALL CONCENTRATIONS OF INSULIN ON FOREARM METABOLISM. PERSISTENCE OF ITS ACTION ON POTASSIUM AND FREE FATTY ACIDS WITHOUT ITS EFFECT ON GLUCOSE. J Clin Invest. 1964 May;43:950–962. doi: 10.1172/JCI104981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierler K. L., Rogus E., Hazlewood C. F. Effect of insulin on potassium flux and water and electrolyte content of muscles from normal and from hypophysectomized rats. J Gen Physiol. 1966 Jan;49(3):433–456. doi: 10.1085/jgp.49.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]


