Abstract
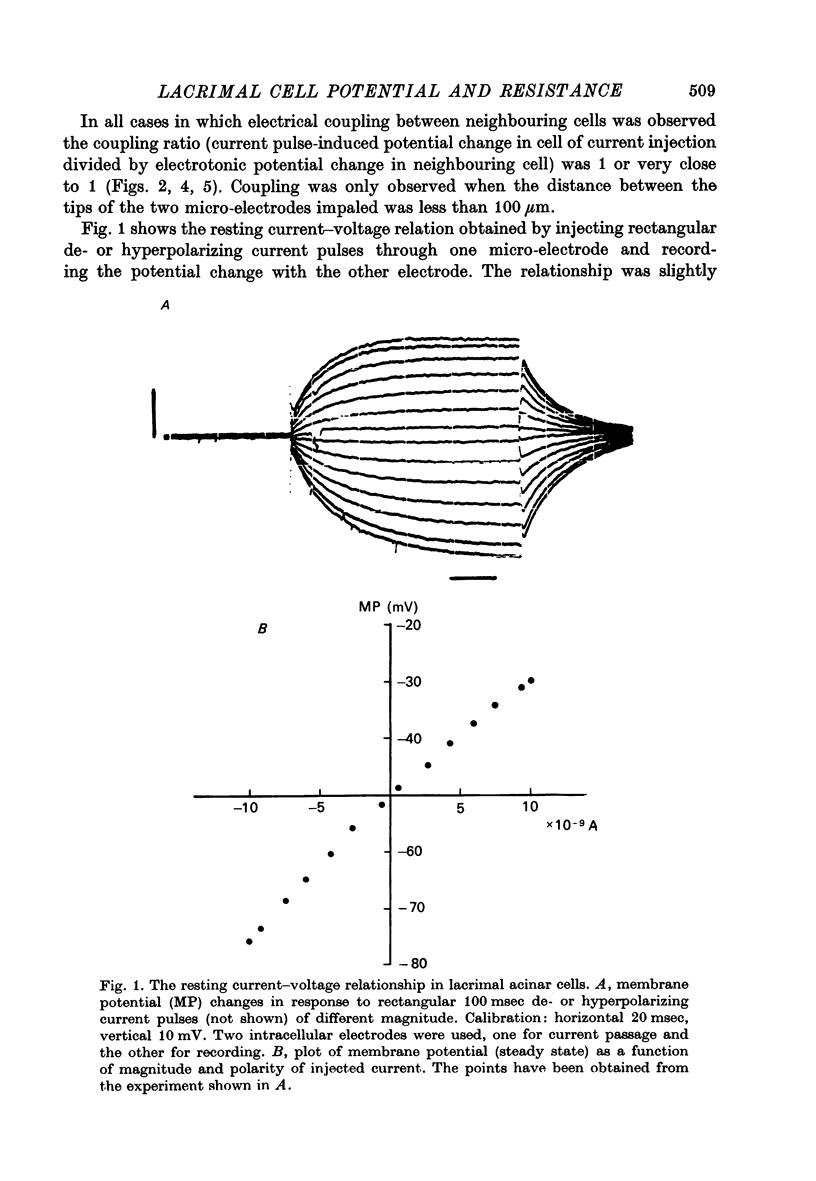
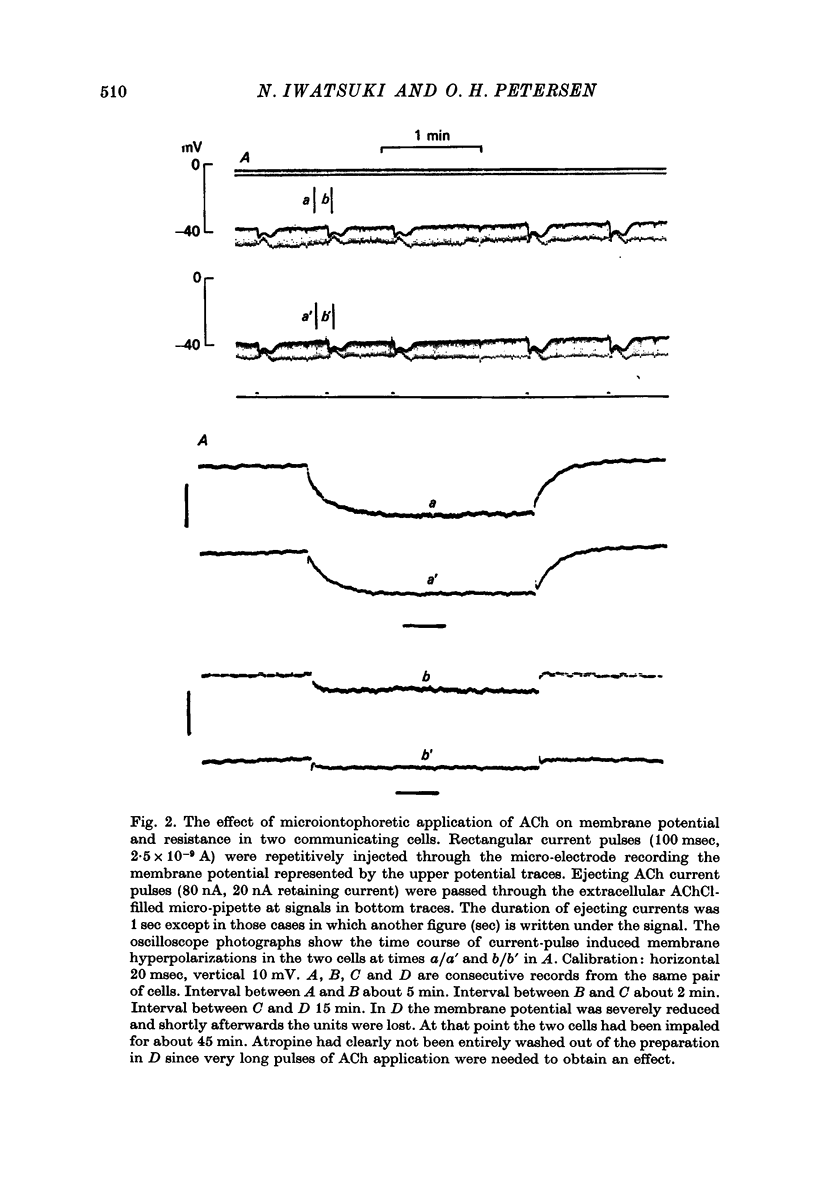
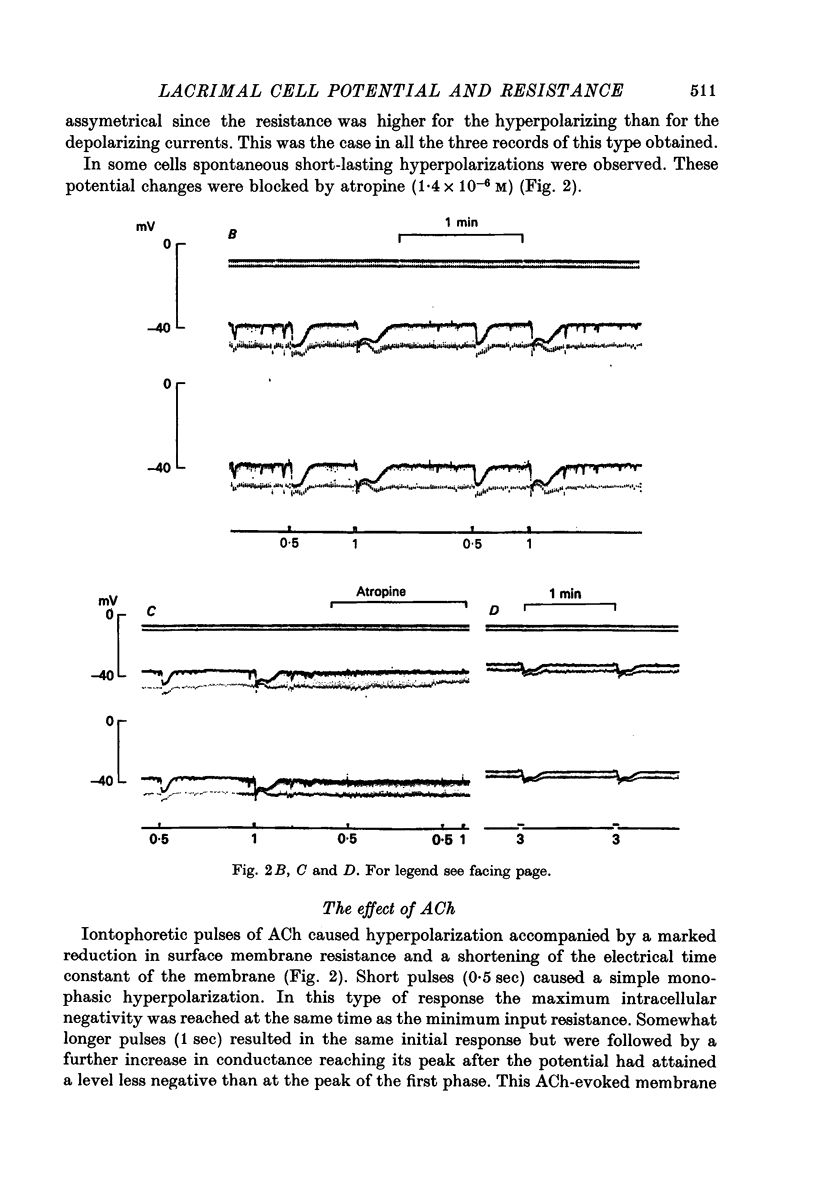
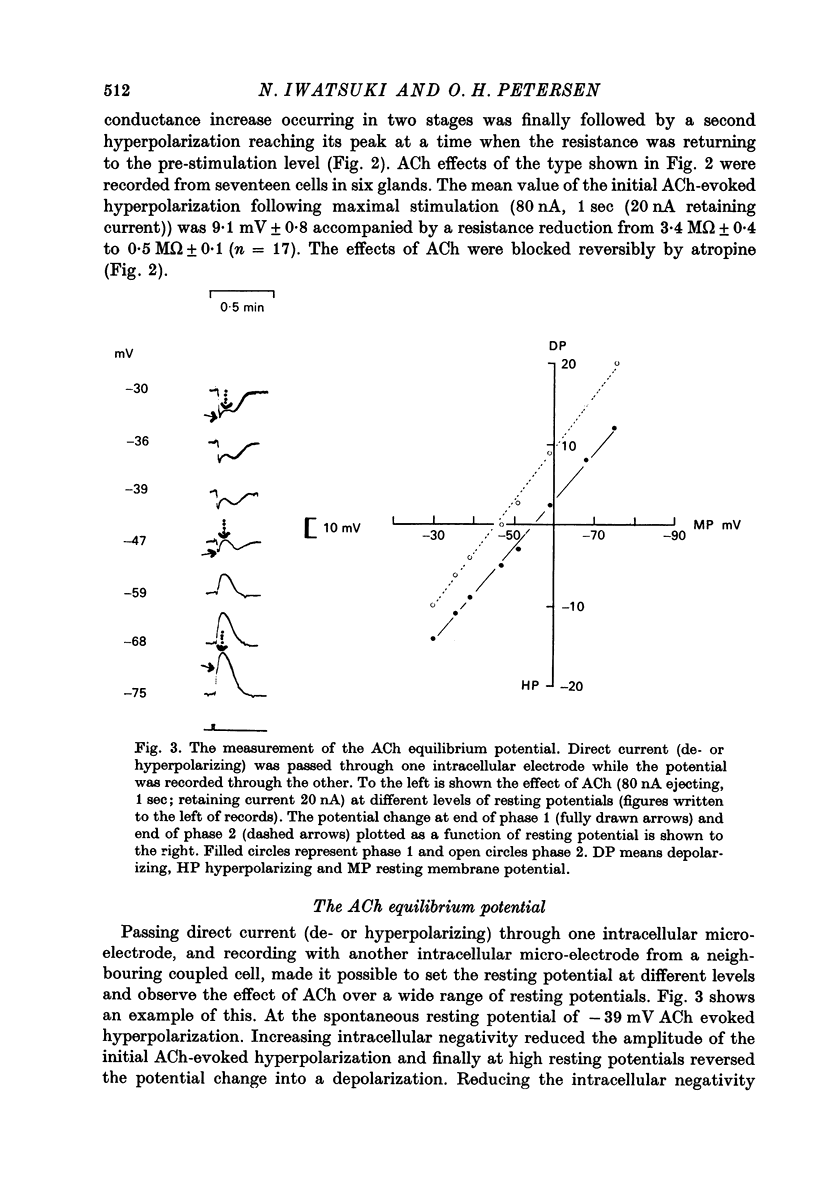
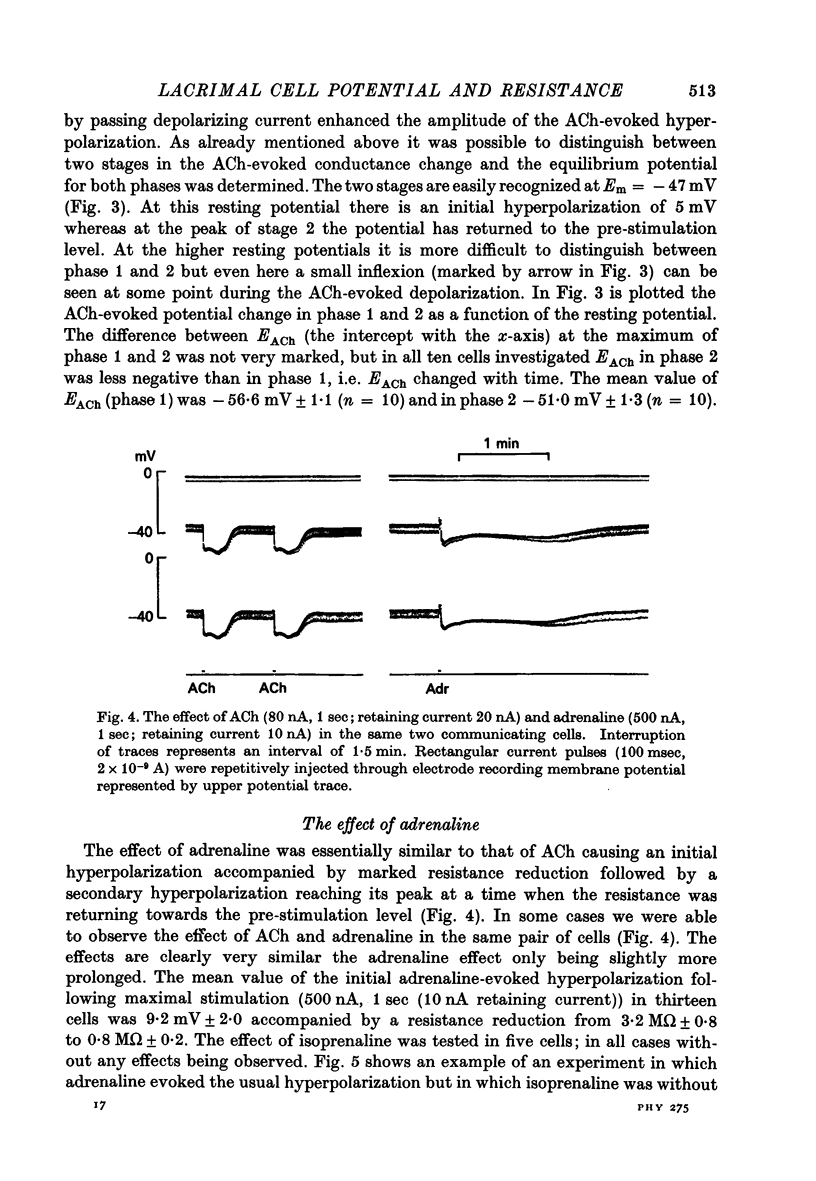
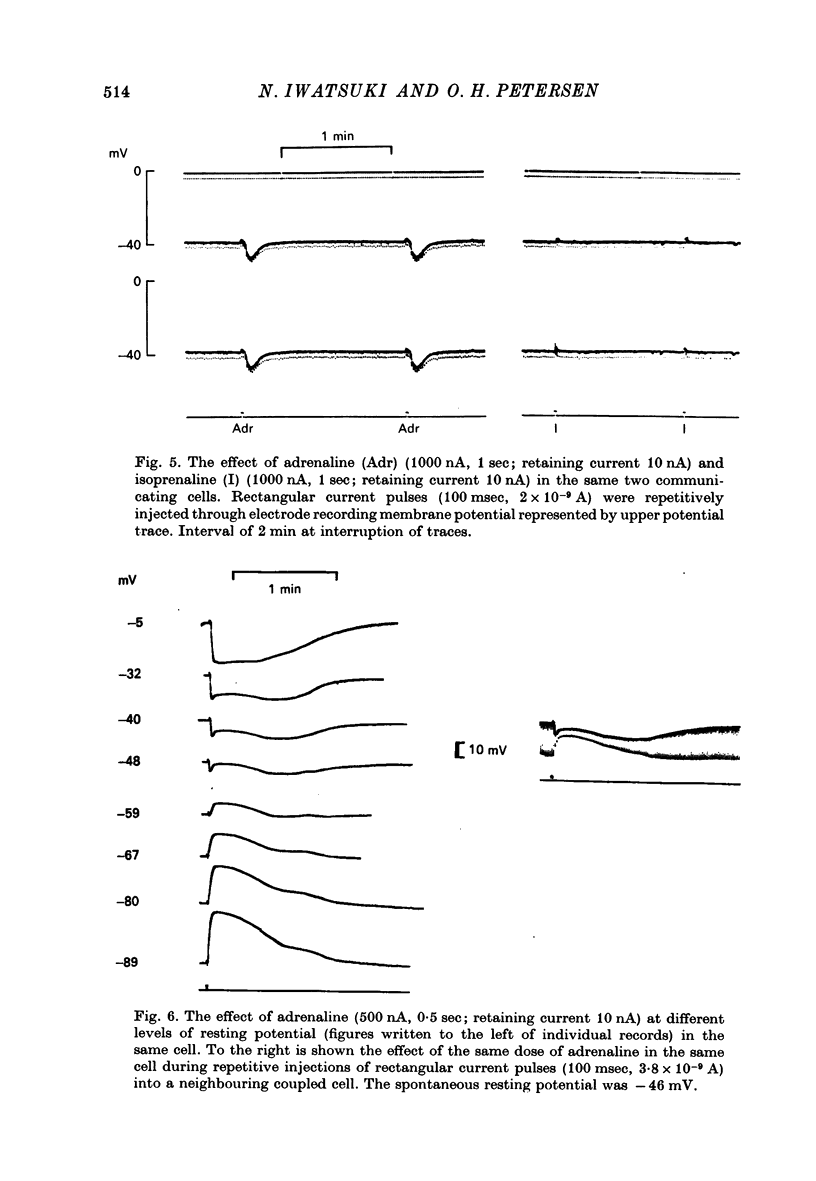
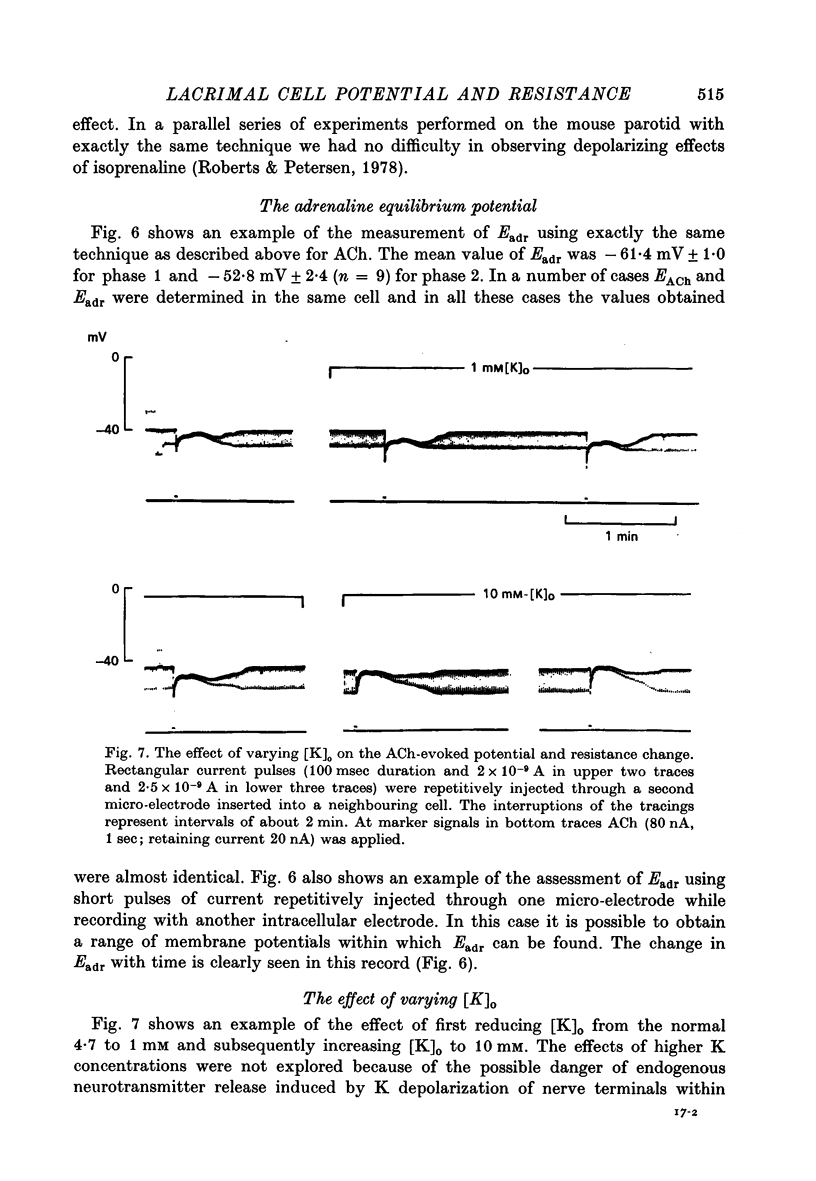
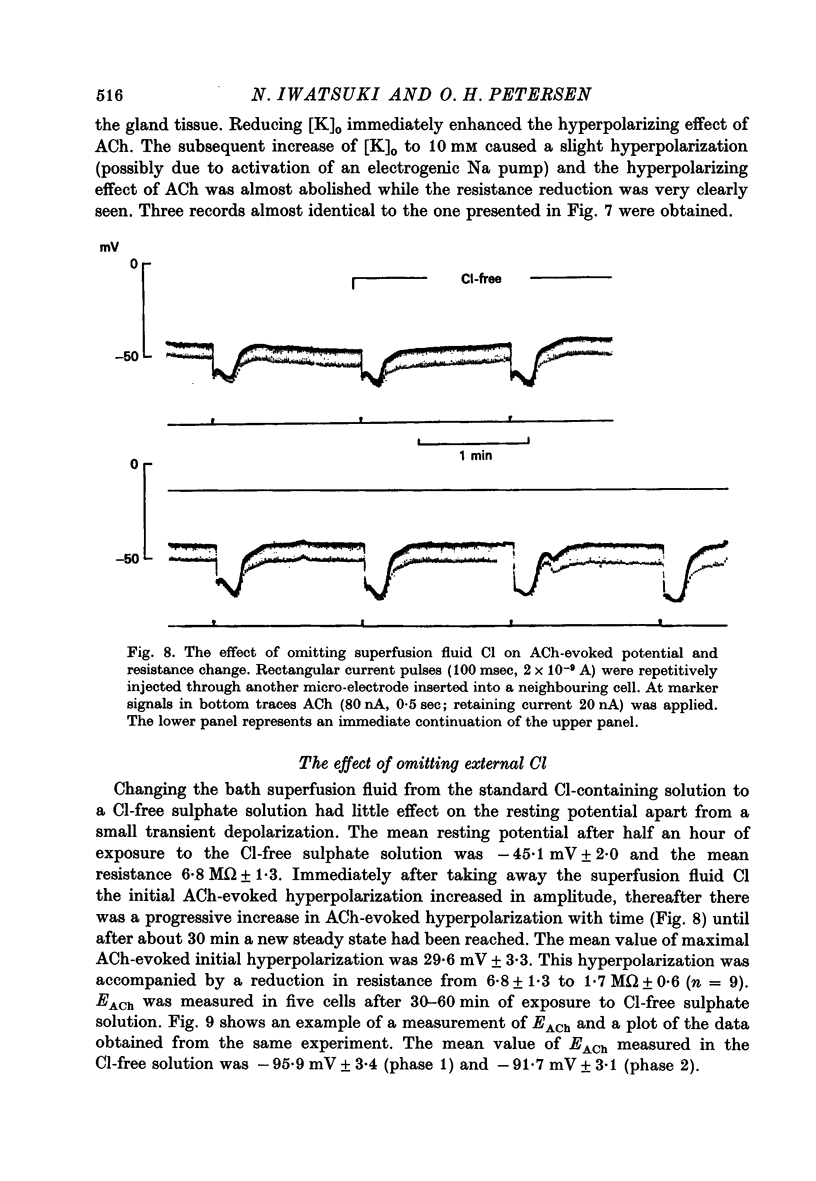
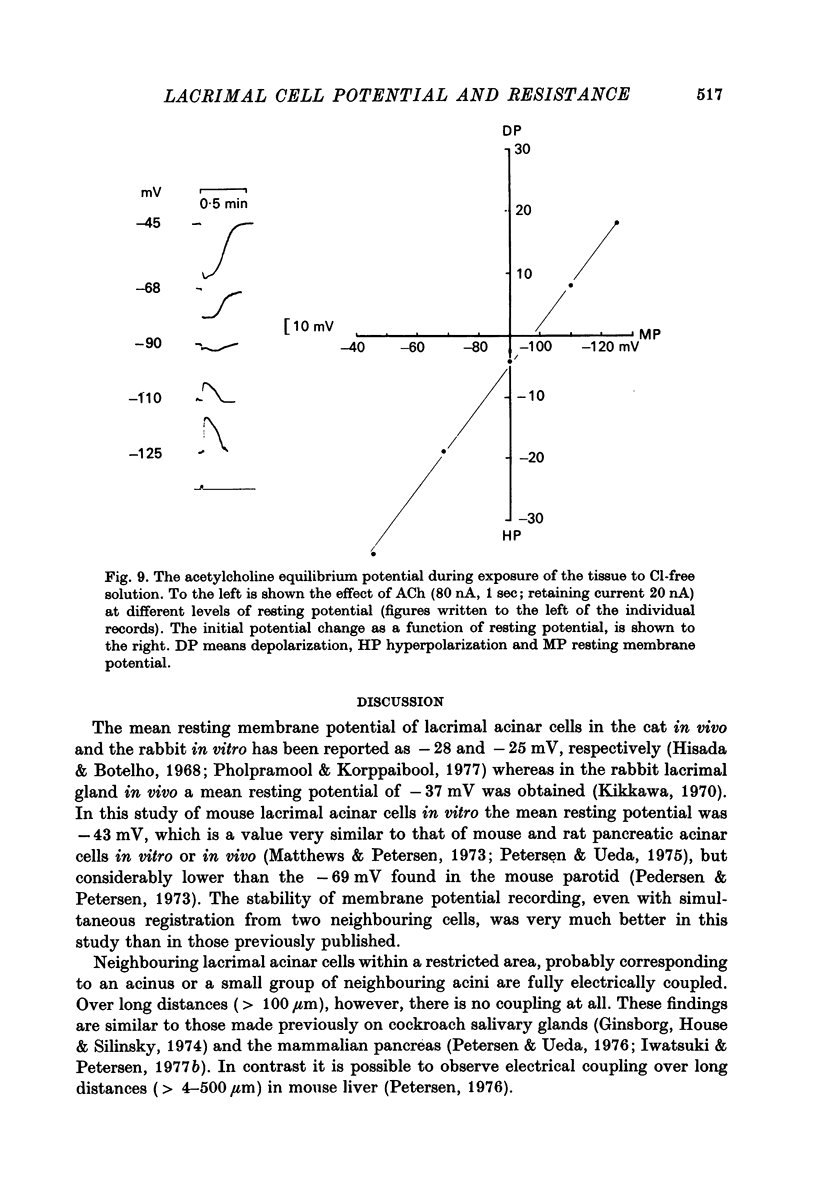
1. Intracellular micro-electrode recordings were made from surface acini of mouse exorbital lacrimal glands placed in a Perspex bath through which oxygenated physiological saline solutions were circulated. Two micro-electrodes were inserted into neighbouring communicating cells. Through one of the electrodes, current pulses could be injected. The cells impaled were stimulated by iontophoresis of acetylcholine (ACh), adrenaline or isoprenaline from an extracellular micropipette. 2. During exposure to standard Krebs solution the resting membrane potential was -42.5 mV +/- 1.2 and the resting input resistance 3.3 Momega +/- 0.3. When the tips of the two intracellular micro-electrodes were more than 100 micrometer apart no electrical coupling between two impaled cells could be detected. At intertip distances below about 80 micrometer coupling was frequently observed. In all such cases the coupling ratio was 1. The resting current-voltage relation was almost linear within the membrane potential range of -30 to -80 mV. 3. During exposure to standard Krebs solution the resting membrane potential was -42.5 mV +/- 1.2 and the resting input resistance 3.3 Momega +/- 0.3. When the tips of the two intracellular micro-electrodes were more than 100 micrometer apart no electrical coupling between two impaled cells could be detected. At intertip distances below about 80 micrometer coupling was frequently observed. In all such cases the coupling ratio was 1. The resting current-voltage relation was almost linear within the membrane potential range of -30 to -80mV. 3. During exposure to standard Krebs solution short iontophoretic pulses of ACh or adrenaline caused fully reversible hyperpolarizations accompanied by marked reduction of surface cell membrane resistance and membrane time constant. The effects of ACh were blocked by atropine (1.4 x 10(-6)M). Iontophoresis of isoprenaline never had any detectable effect on membrane potential or resistance. 4. Applying de- or hyperpolarizing direct currents through one of the two intracellular micro-electrodes the effect of ACh or adrenaline could be observed at different lvels of resting potential. Depolarizing the acinar cell membrane resulted in an enhanced stimulant-evoked hyperpolarization whereas hyperpolarizing the acinar cell membrane resulted in a reduction, and at potentials more negative than -60 mV in a reversal of the stimulant-evoked potential change. The ACh equilibrium potential (EACh) under control conditions was -56.6 mV +/- 1.1 and EAdrenaline was -61.4 mV +/- 1.0. 5. Replacing the control superfusion solution by a Clfree sulphate solution resulted in an immediate shift of EACh towards more negative values. At steady state in the Cl-free solution the resting input resistance was 6.8 Momega +/- 1.3 EACh was -95.9 mV +/- 3.4. 6. Reducing [K]o from the usual 4.7 to 1.0 mM resulted in an immediate marked increase in the amplitude of ACh-evoked hyperpolarization whereas increasing [K]o to 10 mM almost abolished the ACh-evoked potential, but not resistance change. 7...
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. H., van Lennep E. W., Young J. A. Water and electrolyte secretion by the exorbital lacrimal gland of the rat studied by micropuncture and catheterization techniques. Pflugers Arch. 1972;337(4):299–309. doi: 10.1007/BF00586647. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L., House C. R., Silinsky E. M. Conductance changes associated with the secretory potential in the cockroach salivary gland. J Physiol. 1974 Feb;236(3):723–731. doi: 10.1113/jphysiol.1974.sp010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog V., Sies H., Miller F. Exocytosis in secretory cells of rat lacrimal gland. Peroxidase release from lobules and isolated cells upon cholinergic stimulation. J Cell Biol. 1976 Sep;70(3):692–706. doi: 10.1083/jcb.70.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada M., Botelho S. Y. Membrane potentials of in situ lacrimal gland in the cat. Am J Physiol. 1968 Jun;214(6):1262–1267. doi: 10.1152/ajplegacy.1968.214.6.1262. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: localization of acetylcholine receptors and the importance of chloride and calcium for acetylcholine-evoked depolarization. J Physiol. 1977 Aug;269(3):723–733. doi: 10.1113/jphysiol.1977.sp011925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: the acetylcholine equilibrium potential and its ionic dependency. J Physiol. 1977 Aug;269(3):735–751. doi: 10.1113/jphysiol.1977.sp011926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keryer G., Rossignol B. Effect of carbachol on 45Ca uptake and protein secretion in rat lacrimal gland. Am J Physiol. 1976 Jan;230(1):99–104. doi: 10.1152/ajplegacy.1976.230.1.99. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H. Pancreatic acinar cells: ionic dependence of the membrane potential and acetycholine-induced depolarization. J Physiol. 1973 Jun;231(2):283–295. doi: 10.1113/jphysiol.1973.sp010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Membrane potential and resistance measurement in acinar cells from salivary glands in vitro: effect of acetylcholine. J Physiol. 1974 Oct;242(1):173–188. doi: 10.1113/jphysiol.1974.sp010700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: ionic dependence of acetylcholine-induced membrane potential and resistance change. J Physiol. 1975 Jan;244(2):431–465. doi: 10.1113/jphysiol.1975.sp010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: membrane potential and resistance change evoked by acetylcholine. J Physiol. 1974 Apr;238(1):145–158. doi: 10.1113/jphysiol.1974.sp010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen G. L., Petersen O. H. Membrane potential measurement in parotid acinar cells. J Physiol. 1973 Oct;234(1):217–227. doi: 10.1113/jphysiol.1973.sp010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. Electrophysiology of mammalian gland cells. Physiol Rev. 1976 Jul;56(3):535–577. doi: 10.1152/physrev.1976.56.3.535. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Formation of saliva and potassium transport in the perfused cat submandibular gland. J Physiol. 1971 Jul;216(1):129–142. doi: 10.1113/jphysiol.1971.sp009513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. Some factors influencing stimulation-induced release of potassium from the cat submandibular gland to fluid perfused through the gland. J Physiol. 1970 Jun;208(2):431–447. doi: 10.1113/jphysiol.1970.sp009129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Ueda N. Pancreatic acinar cells: effect of acetylcholine, pancreozymin, gastrin and secretin on membrane potential and resistance in vivo and in vitro. J Physiol. 1975 May;247(2):461–471. doi: 10.1113/jphysiol.1975.sp010941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Ueda N. Pancreatic acinar cells: the role of calcium in stimulus-secretion coupling. J Physiol. 1976 Jan;254(3):583–606. doi: 10.1113/jphysiol.1976.sp011248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pholpramool C., Korppaibool S. Ionic dependence of the resting membrane potential of rabbit lacrimal gland in vitro. Biochim Biophys Acta. 1977 Aug 1;468(3):353–363. doi: 10.1016/0005-2736(77)90287-5. [DOI] [PubMed] [Google Scholar]
- Poulsen J. H. Acetylcholine-induced transport of Na+ and K+ in the perfused cat submandibular gland. Pflugers Arch. 1974 Jul 9;349(3):215–220. doi: 10.1007/BF00592449. [DOI] [PubMed] [Google Scholar]
- THAYSEN J. H., THORN N. A. Excretion of urea, sodium, potassium and chloride in human tears. Am J Physiol. 1954 Jul;178(1):160–164. doi: 10.1152/ajplegacy.1954.178.1.160. [DOI] [PubMed] [Google Scholar]


