Abstract
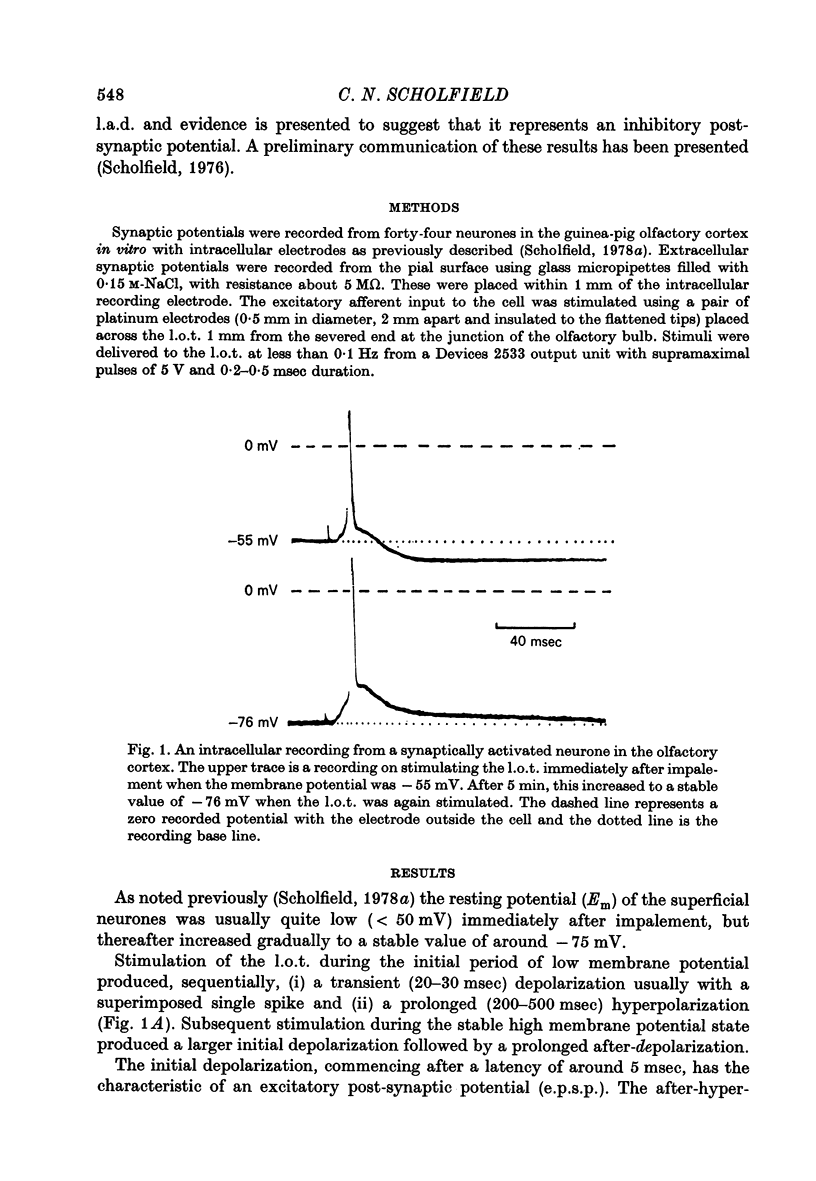
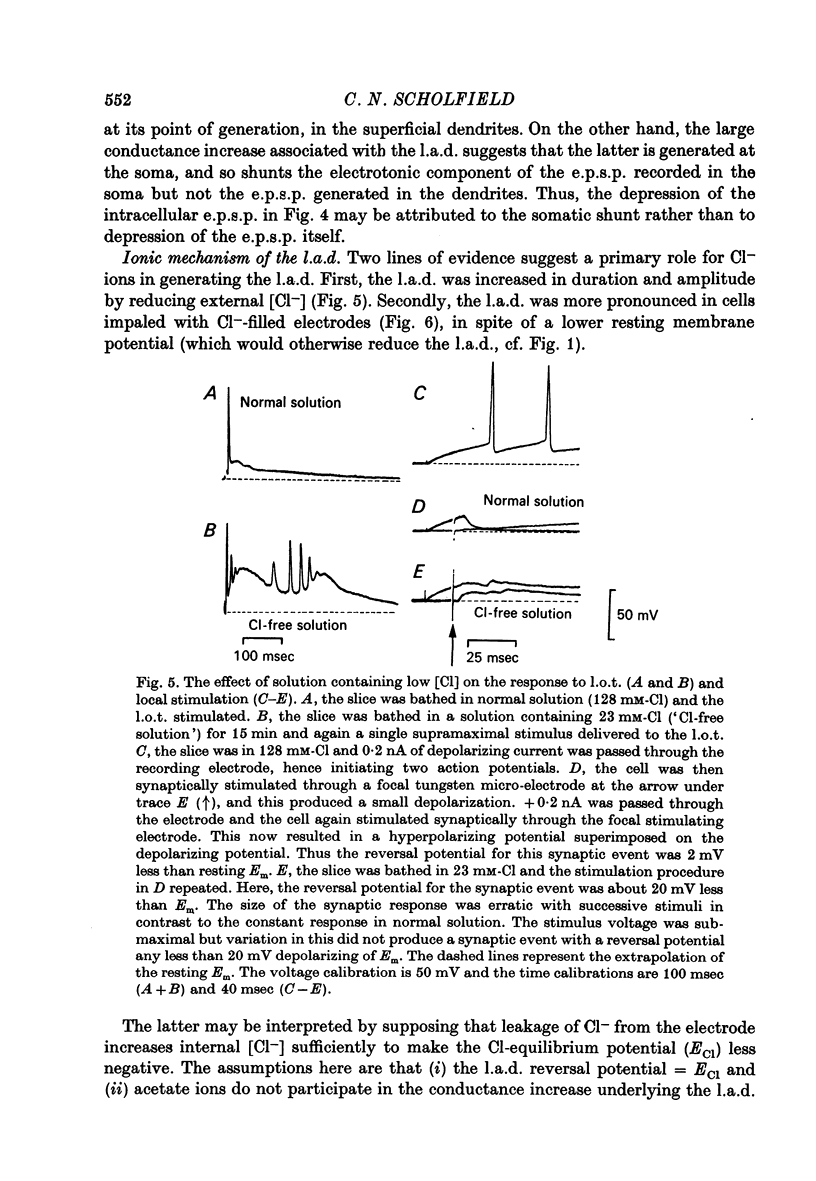
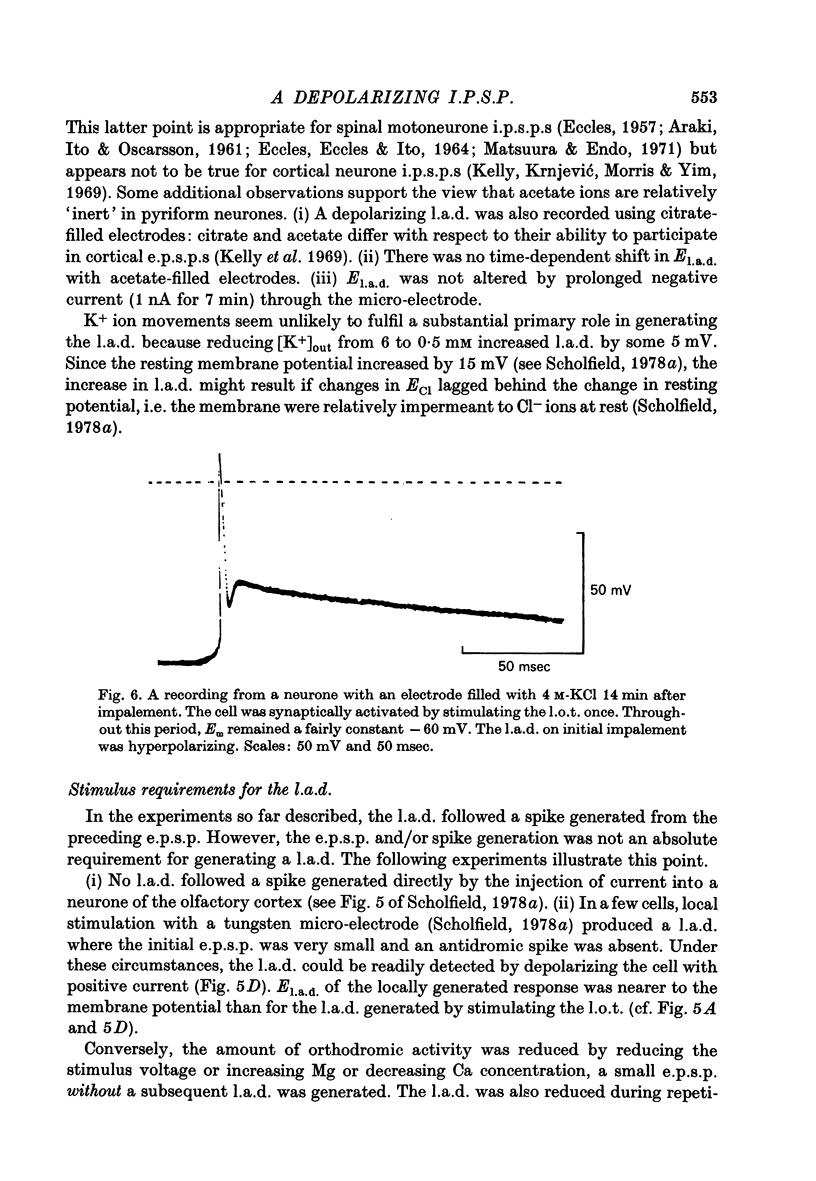
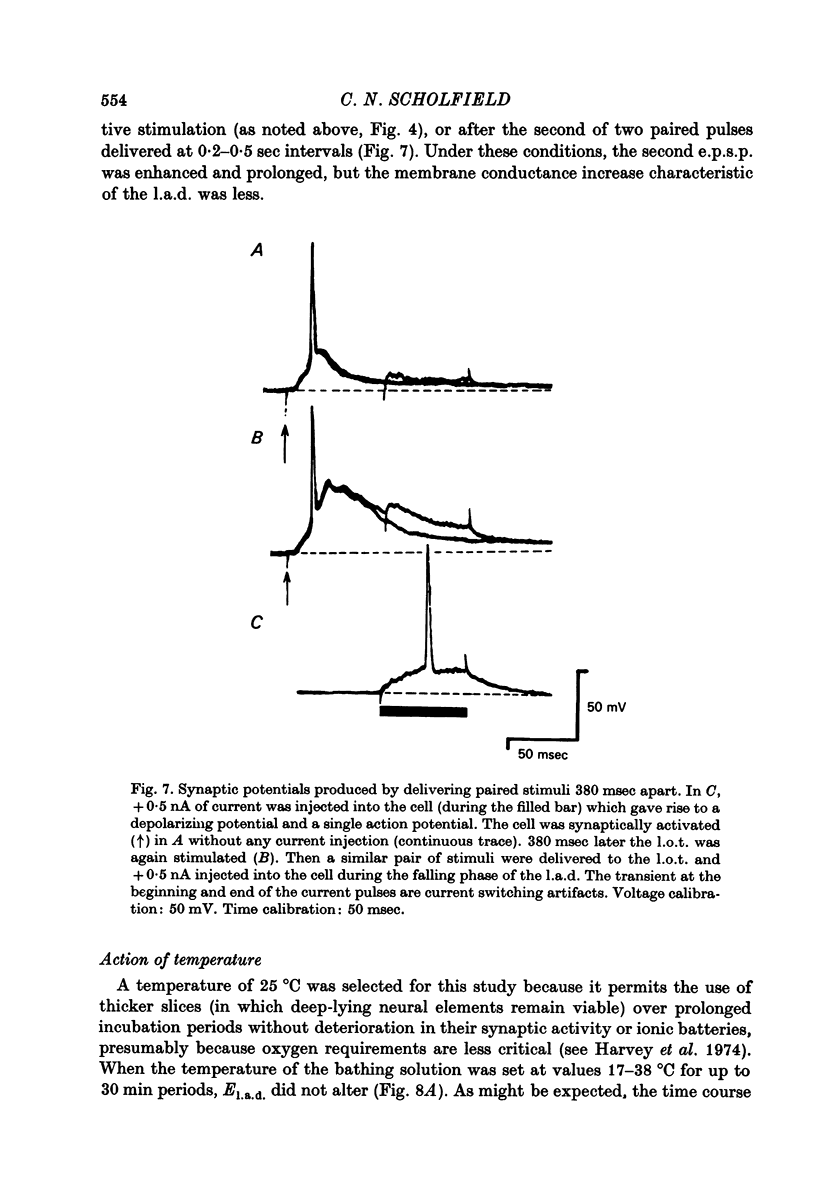
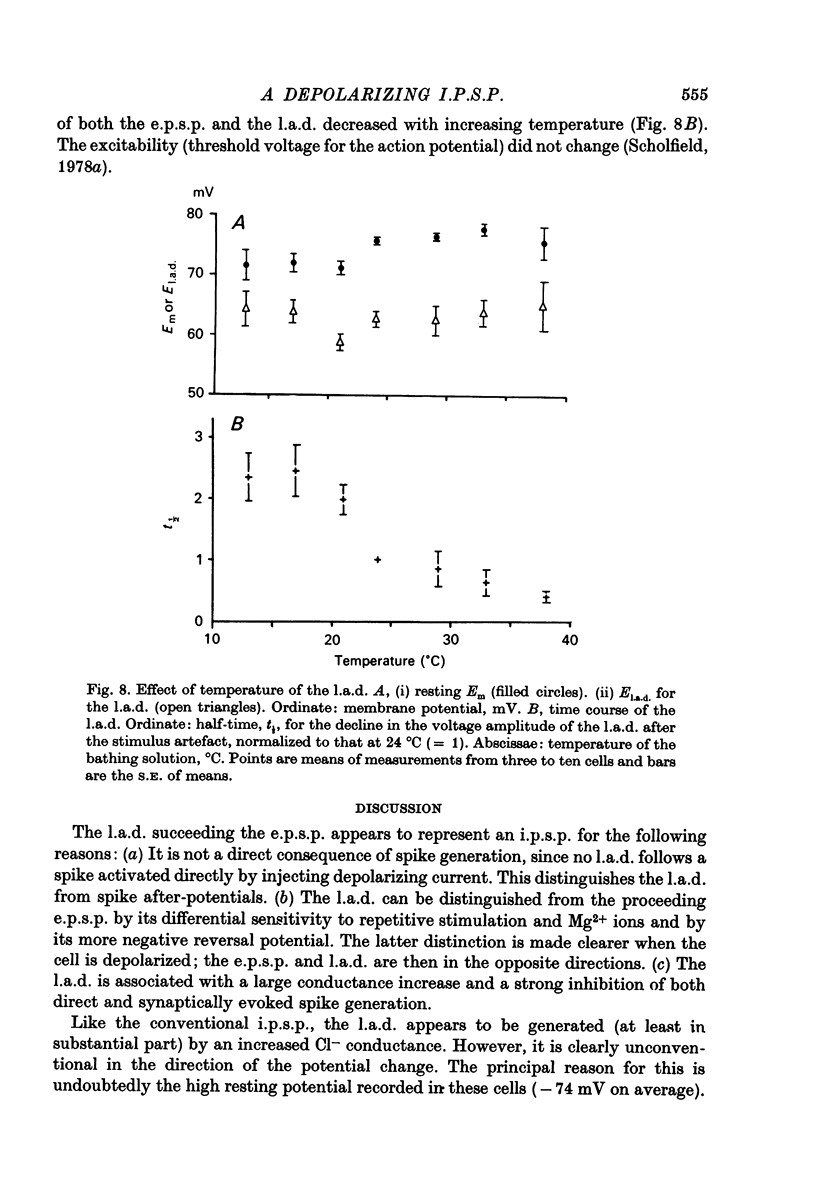
1. Stable intracellular recordings were obtained from neurones in slices of the guinea-pig olfactory cortex maintained in vitro. 2. Single stimuli applied to the lateral olfactory tract (l.o.t.) produced an excitatory post-synaptic potential (e.p.s.p.) usually generating a single spike. 3. The e.p.s.p. was followed by a long (200-500 msec) after-depolarization (l.a.d.) of peak amplitude 5-16 mV. This was accompanied by a very large conductance increase and was associated with an inhibition of the intracellularly recorded e.p.s.p. and of spike generation. 4. The l.a.d. was more susceptible than the e.p.s.p. to depression by (i) repetitive l.o.t. stimulation and (ii) raising external [Mg2+]. The l.a.d. could be generated without a preceding spike. 5. At an average resting membrane potential of -74 mV the average reversal potential for the l.a.d. (El.a.d.) was -63 mV.El.a.d. became more positive on reducing [Cl-]out or on using KCl-filled electrodes. 6. It is concluded that the l.a.d. represents a Cl- -mediated inhibitory post-synaptic potential, generated through deep-lying recurrent inhibitory loops.
Full text
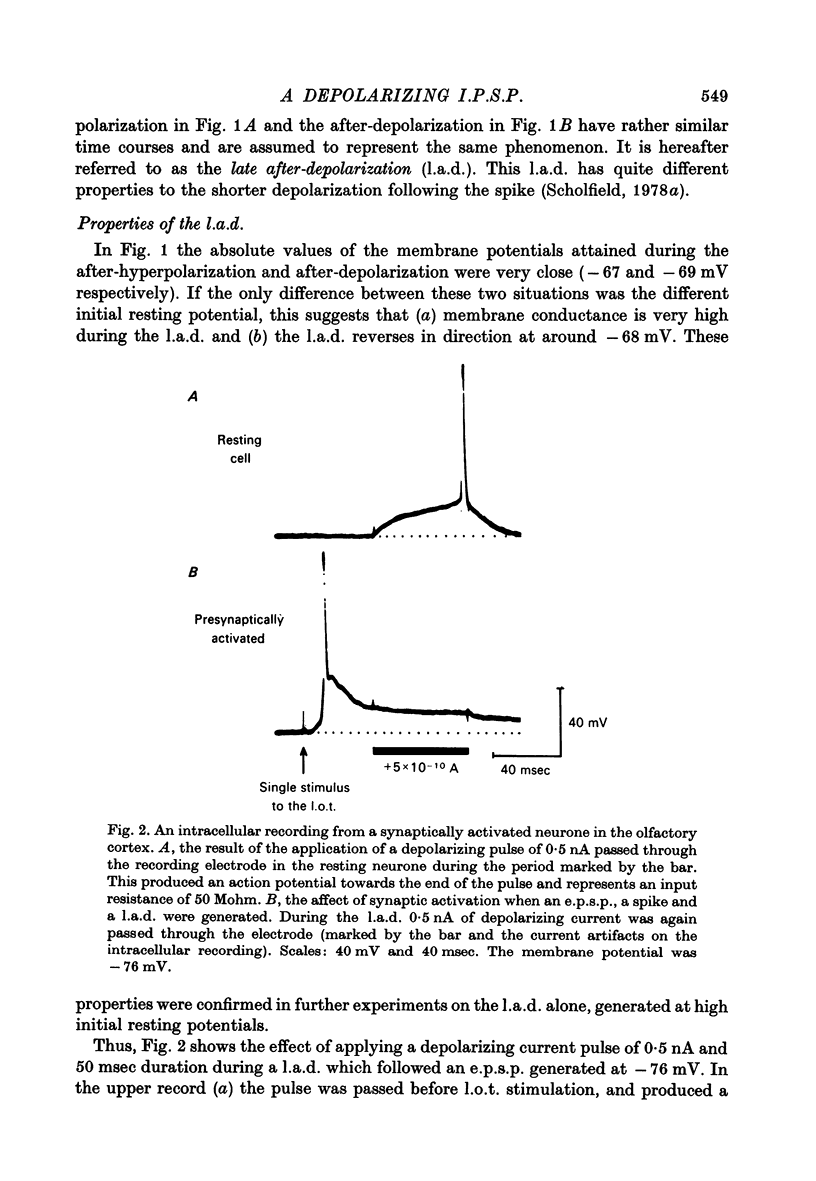
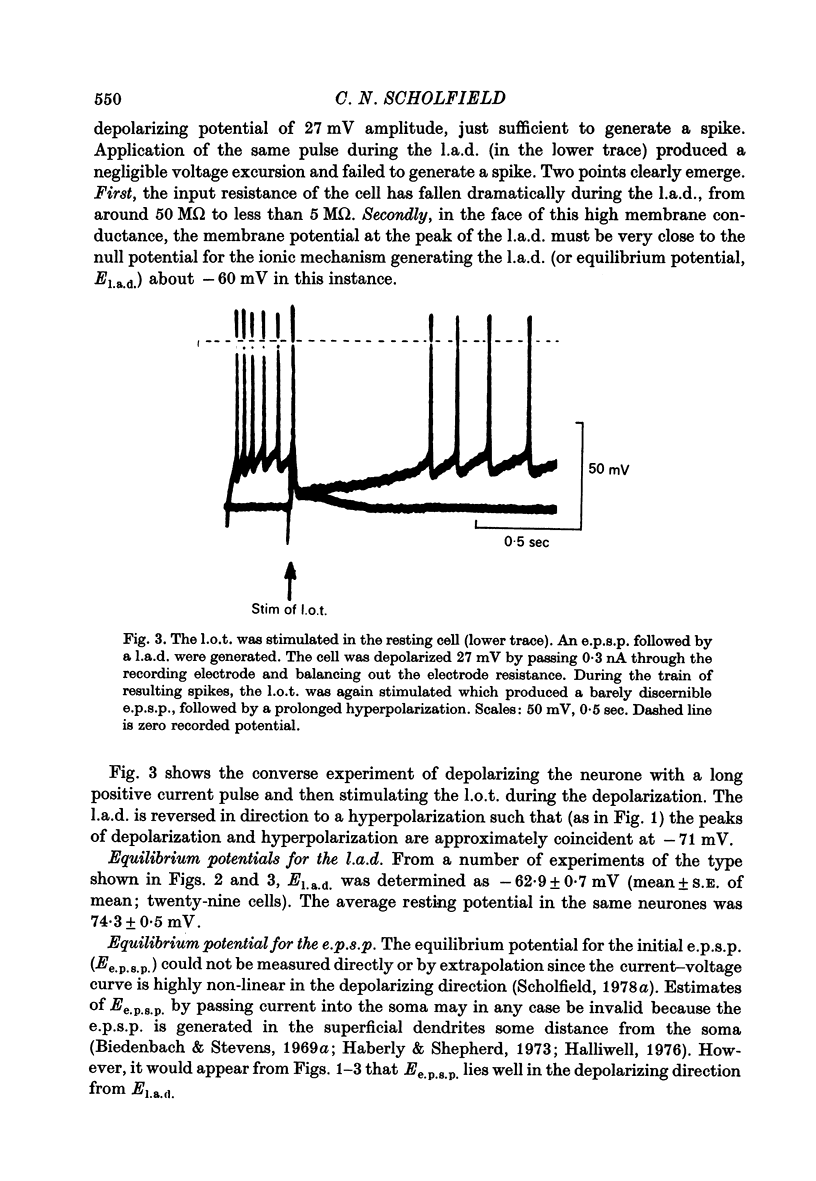
PDF
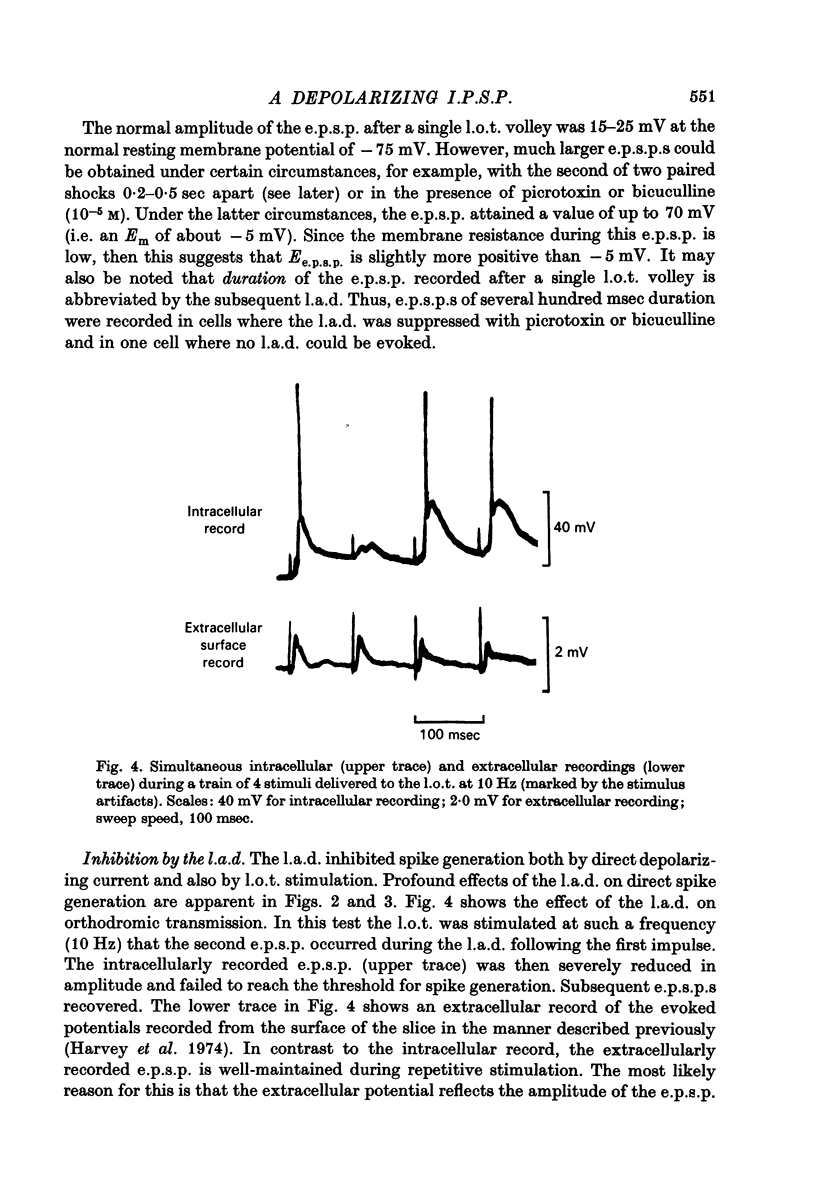


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., ITO M., OSCARSSON O. Anion permeability of the synaptic and non-synaptic motoneurone membrane. J Physiol. 1961 Dec;159:410–435. doi: 10.1113/jphysiol.1961.sp006818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenbach M. A., Stevens C. F. Electrical activity in cat olfactory cortex produced by synchronous orthodromic volleys. J Neurophysiol. 1969 Mar;32(2):193–203. doi: 10.1152/jn.1969.32.2.193. [DOI] [PubMed] [Google Scholar]
- Biedenbach M. A., Stevens C. F. Synaptic organization of cat olfactory cortex as revealed by intracellular recording. J Neurophysiol. 1969 Mar;32(2):204–214. doi: 10.1152/jn.1969.32.2.204. [DOI] [PubMed] [Google Scholar]
- ECCLES J., ECCLES R. M., ITO M. EFFECTS PRODUCED ON INHIBITORY POSTSYNAPTIC POTENTIALS BY THE COUPLED INJECTIONS OF CATIONS AND ANIONS INTO MOTONEURONS. Proc R Soc Lond B Biol Sci. 1964 May 19;160:197–210. doi: 10.1098/rspb.1964.0036. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Haberly L. B., Shepherd G. M. Current-density analysis of summed evoked potentials in opossum prepyriform cortex. J Neurophysiol. 1973 Jul;36(4):789–802. doi: 10.1152/jn.1973.36.4.789. [DOI] [PubMed] [Google Scholar]
- Harvey J. A., Scholfield C. N., Brown D. A. Evoked surface-positive potentials in isolated mammalian olfactory cortex. Brain Res. 1974 Aug 16;76(2):235–245. doi: 10.1016/0006-8993(74)90457-0. [DOI] [PubMed] [Google Scholar]
- Kelly J. S., Krnjević K., Morris M. E., Yim G. K. Anionic permeability of cortical neurones. Exp Brain Res. 1969;7(1):11–31. doi: 10.1007/BF00236105. [DOI] [PubMed] [Google Scholar]
- Matsuura S., Endo K. Anion permeability of the inhibitory subsynaptic membrane of the spinal motoneuron of the toad. Jpn J Physiol. 1971 Jun;21(3):265–276. doi: 10.2170/jjphysiol.21.265. [DOI] [PubMed] [Google Scholar]
- Nishi S., Minota S., Karczmar A. G. Primary afferent neurones: the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974 Mar;13(3):215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Obata K. Transmitter sensitivities of some nerve and muscle cells in culture. Brain Res. 1974 Jun 14;73(1):71–88. doi: 10.1016/0006-8993(74)91008-7. [DOI] [PubMed] [Google Scholar]
- Pickles H. G., Simmonds M. A. Field potentials, inhibition and the effect of pentobarbitone in the rat olfactory cortex slice. J Physiol. 1978 Feb;275:135–148. doi: 10.1113/jphysiol.1978.sp012181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D., Sercombe R. Electrical activity observed in guinea-pig olfactory cortex maintained in vitro. J Physiol. 1968 Aug;197(3):667–683. doi: 10.1113/jphysiol.1968.sp008581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield C. N. A barbiturate induced intensification of the inhibitory potential in slices of guinea-pig olfactory cortex. J Physiol. 1978 Feb;275:559–566. doi: 10.1113/jphysiol.1978.sp012208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield C. N. Electrical properties of neurones in the olfactory cortex slice in vitro. J Physiol. 1978 Feb;275:535–546. doi: 10.1113/jphysiol.1978.sp012206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F. Structure of cat frontal olfactory cortex. J Neurophysiol. 1969 Mar;32(2):184–192. doi: 10.1152/jn.1969.32.2.184. [DOI] [PubMed] [Google Scholar]
- Yamamoto C., McIlwain H. Electrical activities in thin sections from the mammalian brain maintained in chemically-defined media in vitro. J Neurochem. 1966 Dec;13(12):1333–1343. doi: 10.1111/j.1471-4159.1966.tb04296.x. [DOI] [PubMed] [Google Scholar]


