Abstract
The human facultative pathogenic yeast Candida albicans causes mucocutaneous infections and is the major cause of opportunistic fungal infections in immunocompromised patients. C. albicans activates both the alternative and classical pathway of the complement system. The aim of this study was to assay whether C. albicans binds human complement regulators in order to control complement activation at its surface. We observed binding of two central complement regulators, factor H and FHL-1, from normal human serum to C. albicans by adsorption assays, immunostaining, and fluorescence-activated cell sorter (FACS) analyses. Specificity of acquisition was further confirmed in direct binding assays with purified proteins. The surface-attached regulators maintained their complement regulatory activities and mediated factor I-dependent cleavage of C3b. Adsorption assays with recombinant deletion mutant proteins were used to identify binding domains. Two binding sites were localized. One binding domain common to both factor H and FHL-1 is located in the N-terminal short consensus repeat domains (SCRs) 6 and 7, and the other one located in C-terminal SCRs 19 and 20 is unique to factor H. These data indicate that by surface acquisition of host complement regulators, the human pathogenic yeast C. albicans is able to regulate alternative complement activation at its surface and to inactivate toxic complement activation products.
Candida albicans is the most common human facultative pathogenic yeast and causes disseminated infections and causes opportunistic infections of cutaneous and mucocutaneous surfaces (3, 37). As a yeast form it is a common saprophyte in healthy individuals and resides mainly on the skin and in the oral cavity and urogenital and gastrointestinal tracts. In addition, it is also a causative agent of systemic infections in immunocompromised patients, especially in granulocytopenic patients (12). C. albicans infections, which are difficult to diagnose and treat, can be lethal (10). The ability to invade host tissues is an essential part of the pathogenicity of C. albicans and is mediated via surface-expressed adhesion molecules, especially mannoproteins (5) and secretory proteolytic enzymes (4). Also, morphogenetic changes from yeast to hyphal forms increase adherence to host cells and penetration of tissue and are therefore an essential part of the infection process (7).
The complement system is an important part of innate immunity. The alternative pathway (AP) is activated on nonprotected surfaces and plays a pivotal role in the clearance of microorganisms. C3 is a central component of the cascade. Cleavage products of C3b on microbial surfaces act as opsonins for neutrophils, macrophages, and eosinophils. Further activation of the terminal pathway leads to formation of potentially cytolytic membrane attack complexes (MAC) on the target surfaces. The complement activation system is controlled by several fluid-phase and cell surface regulators. The main fluid-phase regulators of the AP are factor H and FHL-1. The latter is the product of an alternatively processed nuclear RNA transcript of the factor H gene (15, 46). Both proteins are exclusively composed of homologous short consensus repeat domains (SCRs), each with approximately 60 amino acids. Factor H, with a molecular mass of 150 kDa, consists of 20 SCRs, and FHL-1, with a molecular mass of 42 kDa, is composed of 7 SCRs identical to the N-terminal SCRs of factor H. In addition, the FHL-1 protein has a unique C-terminal extension of four amino acids. Both proteins act as cofactors for the plasma serine protease factor I in the cleavage of C3b (30, 33, 36) and accelerate the decay of the C3 convertase, C3bBb (32, 43). Factor H and FHL-1 can also compete with factor B in binding to intact C3b (11). These regulatory functions lead to downregulation or termination of the complement cascade.
Several pathogenic microbes have been shown to utilize host complement regulators for immune evasion and downregulation of complement activation. Streptococcus pyogenes (22, 24, 27), Streptococcus pneumoniae (34), Neisseria gonorrhoeae (39), Neisseria meningitidis (38), Borrelia burgdorferi (2, 21, 29), Echinococcus granulosus (8), and the human immunodeficiency virus (42) have been reported to bind factor H and/or FHL-1. In their bound configuration these regulators maintain their complement regulatory activities and thus protect microbes against complement-mediated phagocytosis and direct lysis.
C. albicans activates both the alternative and classical pathways of complement (28). C3b molecules bind directly to the C. albicans surface (AP activation) or via mannan-specific immunoglobulin G antibodies, which occur naturally in human serum (classical pathway activation) (44). In light of the multiple mechanisms whereby the complement system is activated by C. albicans, it is unclear how this yeast controls complement activation products and whether such products mediate and facilitate opsonization.
In this study we examined utilization of complement regulators factor H and FHL-1 by the yeast form of C. albicans and show binding of factor H and FHL-1 from human serum, as well as purified native and recombinant proteins, to the surface of C. albicans. One binding site, which is common to factor H and FHL-1, was localized within SCRs 6 and 7, and a second site, which is unique to factor H, is within the two most-C-terminal domains of factor H. Attachment via these sites maintain the regulatory functions and allow the inactivation of complement activation products directly on the C. albicans surface.
MATERIALS AND METHODS
C. albicans strains and growth conditions.
The wild-type SC5314 (14) strain of C. albicans was used in all experiments. C. albicans and the other analyzed strains Candida glabrata, Candida krusei, Candida tropicalis, and Candida parapsilosis were grown at 28°C on a shaker for 16 h in YPD medium (2% glucose, 2% peptone, 1% yeast extract). After centrifugation (5 min at 3,838 × g) the cells were washed with Veronal-buffered saline (VBS) (150 mM NaCl, 3.3 mM diethyl barbiturate, pH 7.5) and counted with a cell counter (Beckman Coulter, Krefeld, Germany). Candida cells were also incubated at 37°C in the presence of 10% N-acetylglucosamine.
Serum, antibodies, and proteins.
Pooled normal human sera (NHS) were obtained from healthy human donors from the blood bank of the University Clinics of the Friedrich Schiller University, Jena, Germany, upon informed consent and stored at −80°C until used. EDTA was added at a concentration of 10 mM (NHS-EDTA).
Antibodies used in experiments were polyclonal rabbit-anti-SCRs 1 to 4 for the detection of the N-terminal SCRs of factor H and FHL-1 and rabbit polyclonal antiserum for the detection of the C-terminal SCRs 19 and 20 of factor H. A polyclonal goat anti-factor H antiserum was obtained from Calbiochem (La Jolla, Calif.). Fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antiserum and the horseradish peroxidase-conjugated rabbit anti-goat antiserum were from Dako (Glostrup, Denmark).
Factor H and factor I were obtained from Calbiochem. C3 was purified from the NHS pool, and C3b was generated by incubation with factors B and D in the presence of Ni2+ or Mg2+ ions as described previously (26).
Expression of recombinant proteins.
Recombinant deletion mutants of factor H (SCRs 1 to 5,1 to 6, 8 to 20, 8 to 11, 15 to 18, 15 to 20, and 19 and 20) and FHL-1 (SCRs 1 to 7) were expressed in the baculovirus system as described elsewhere (31). Shortly, Spodoptera frugiperda (Sf9) cells were grown in expression medium (BioWhittaker, Verviers, Belgium) supplemented with streptomycin (100 μg/ml), penicillin (100 U/ml), and amphotericin B (250 ng/ml) and infected with a recombinant baculovirus at a multiplicity of infection of 5. The culture supernatant was collected 9 days after infection, and recombinant proteins were purified by Ni+-chelate chromatography as described elsewhere (31) or by Äkta fast-performance liquid chromatography purification (Pharmacia, Piscataway, N.J.). The proteins were concentrated using Centricon microconcentrators with a cutoff at 10 kDa (Millipore, Bedford, Mass.).
Immunofluorescence assays and FACS analyses.
C. albicans (5 × 107 cells) was incubated at 4°C for 4 h with NHS-EDTA. After incubation the cells were washed three times with ice-cold PBS (0.15 M NaCl, 0.03 M phosphate [pH 7.2]), and nonspecific binding sites were blocked with 1% nonfat dry milk-PBS for 30 min at 4°C. The samples were incubated with rabbit anti-SCRs 1 to 4 or anti-SCRs 19 and 20 antiserum for 2 h at 4°C (1:50 dilution). After three washes with PBS the secondary FITC-conjugated goat anti-rabbit immunoglobulin G was added at a dilution of 1:50 in 1% bovine serum albumin (BSA)-PBS. The cells were washed three times with cold PBS and the samples were examined with an Olympus BX51 microscope (Olympus Optical, Tokyo, Japan) equipped with a filter specific for fluorescein isothiocyanate. An Olympus ColorView digital camera and Analysis software (Soft Imaging System, Münster, Germany) were used for photographing. The labeled cells were also examined by FACS (FACScan; Becton Dickinson). Forward and sideward scatters were used to define the fluorescent cell population, and 10,000 events were routinely counted.
Serum absorption experiments.
C. albicans cells (5 × 109) were incubated in NHS-EDTA, with concentrated recombinant deletion mutants of factor H and FHL-1 (SCRs 1 to 5, 1 to 6, 8 to 20, 8 to 11, 15 to 18, 15 to 20 or 19 and 20) or with recombinant FHL-1 for 60 min at room temperature (RT). The cells were washed five times with PBS-T (0.15 M NaCl, 0.03 M phosphate, 0.05% Tween [pH 7.2]). Proteins bound to the surfaces of the cells were eluted with 0.1 M glycine-HCl, pH 2.0, and the supernatants were collected.
Samples of the wash and elution fractions were subjected to a nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. The membranes were blocked with 2.5% BSA-PBS-0.1% Tween-10% RotiBlock (Carl Roth, Karlsruhe, Germany) for 12 h at 4°C and incubated further with a rabbit polyclonal anti-factor H antiserum (dilution, 1:1,000) for 120 min at RT. After five washes with 0.1%Tween-PBS, horseradish peroxidase-conjugated rabbit anti-goat antibody was added at a dilution of 1:2,000, and the membranes were incubated at RT for 60 min. After five washes with 0.1% Tween-PBS, the proteins were detected by enhanced chemiluminescence (Amersham Pharmacia, Piscataway, N.J.).
Protein binding assays.
Factor H (Calbiochem), purified recombinant FHL-1, C3b purified from NHS, and BSA were labeled with 125I using the Iodogen technique (Pierce Chemical Corp., Rockford, Ill.) (41). C. albicans cells grown either at 28 or 37°C were washed three times with 1/3 × VBS (VBS with 50 mM NaCl). Cells (107, 108, and 109) were incubated with the radiolabeled proteins (20,000 cpm/assay) in 1/3 GVBS (VBS containing 0.1% gelatin) for 20 min at 37°C. In competition assays nonlabeled purified factor H was added to the reaction mixture. Cell-associated and free radioactive proteins were separated by centrifuging the samples through a 300-μl column of 20% (wt/vol) sucrose in 1/3 GVBS. Radioactivity in the supernatant and the pellet fraction was measured, and the amounts of bound proteins were calculated as percentages of the total radioactivity. All experiments were performed in quadruplicate.
Effect of heparin on factor H binding to C. albicans.
The effect of heparin on the binding of factor H to C. albicans was assayed by incubating cells in the presence of the indicated concentrations (0 to 1,000 IU/ml) of heparin together with labeled factor H. Binding of factor H was detected as described in the protein binding assay. Heparin (5,000 IU/ml) was from Lövens Kemiske Fabrik, Ballerup, Denmark.
Cofactor assay.
Cofactor activity assay of surface-attached regulators was performed as described previously (20). The cells (107) were washed three times with VBS and incubated with NHS-EDTA (both at a dilution of 1:2), purified factor H (100 μg/ml), or VBS for 30 min at 37°C on a shaker. Cells were washed three times with VBS, radiolabeled 125I-C3b (50,000 cpm/assay) and factor I (50 ng/reaction mixture) were added, and the cells were further incubated for 60 min at 37°C. The samples were centrifuged, and the supernatants and pellets were analyzed by SDS-PAGE under reducing conditions to detect the cleavage of 125I-C3b. As a positive control purified factor H (50 ng) was added to the reaction mixture. As a negative control 125I-C3b was incubated in the presence of factor I. The gels were fixed with 5% acetic acid for 10 min, dried and autoradiographed.
RESULTS
Absorption of factor H and FHL-1 from human serum by C. albicans.
To determine whether C. albicans binds the human complement regulators factor H and FHL-1, yeast cells were incubated in NHS-EDTA, and after extensive washing the absorbed proteins were eluted. Wash and elution fractions were separated by SDS-PAGE and analyzed by Western blotting using a goat anti-factor H antiserum, which detects both the 150-kDa factor H and the 42-kDa FHL-1 protein. The presence of both factor H and FHL-1 in the elution fractions shows that C. albicans is able to acquire both human complement regulators from NHS (Fig. 1, lane 2).
FIG. 1.
Binding of human complement regulators factor H and FHL-1 by C. albicans. Fungal cells (109) were incubated in NHS-EDTA and washed, and bound proteins were eluted with 0.1 M glycine. The wash (W) (lane 1) and elution (E) (lane 2) fractions were separated by SDS-PAGE and analyzed by Western blotting using a goat anti-factor H antiserum recognizing both factor H and FHL-1. A control in which factor H and FHL-1 were detected in human serum with the same antiserum is shown in lane 3.
Binding of factor H and FHL-1 from NHS to intact cells of C. albicans.
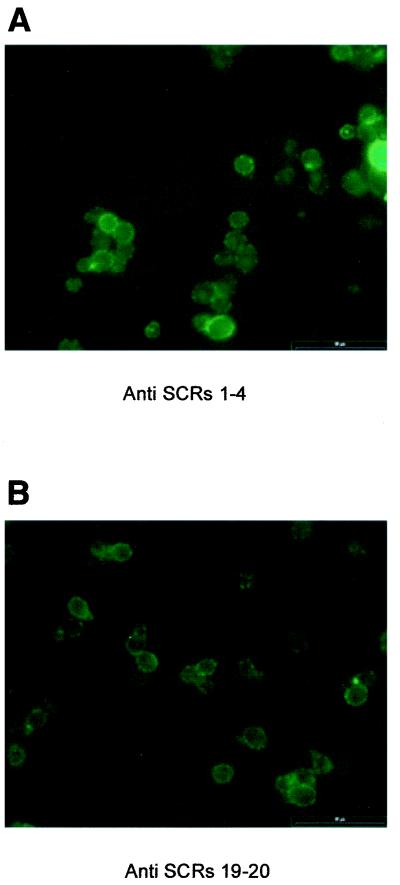
Binding of factor H and FHL-1 to intact C. albicans and the surface distribution of the absorbed immune regulators were analyzed by immunofluorescence microscopy. Cells were incubated in NHS-EDTA first and after thorough washing were stained with the appropriate antibody. As there is no antibody available for the specific detection of FHL-1, we used a subtractive approach to assay the binding of FHL-1. Antiserum against SCRs 1 to 4 (detecting both factor H and FHL-1 at their common N-terminal SCRs) as well as antiserum against SCRs 19 and 20 (specific for factor H recognizing the C-terminal SCRs) were used. The clear positive staining with anti-SCR 1 to 4 antiserum shows binding of factor H and FHL-1 to C. albicans (Fig. 2A). When antiserum specific for factor H (anti-SCRs 19 and 20) was used, staining was evident but less intense (Fig. 2B). The bound proteins showed a rather patchy surface distribution, suggesting a clustering of the ligand molecules on the surface of C. albicans. Incubation with the secondary-conjugated antibody alone after incubation in NHS-EDTA and incubation in buffer instead of NHS-EDTA (data not shown) were performed as control experiments. No positive staining was observed in either case.
FIG. 2.
Immunofluorescence analysis of factor H and FHL-1 binding to intact C. albicans. Cells were incubated in NHS-EDTA, washed, and incubated further with a polyclonal rabbit antiserum detecting common epitopes of factor H and FHL-1 (i.e., anti-SCRs 1 to 4) (A) or with a polyclonal rabbit antiserum specific for factor H (anti-SCRs 19 and 20) (B). Bound antibodies were detected with a FITC-conjugated goat anti-rabbit antibody.
To quantify the binding of factor H and FHL-1 from NHS-EDTA and to further verify the results attachment was also analyzed by flow cytometry. Using an antiserum detecting both factor H and FHL-1 (anti-SCRs 1 to 4) binding of factor H and FHL-1 to C. albicans was observed (Fig. 3A). When antiserum specific for factor H (anti-SCRs 19 and 20) was used, binding was clearly positive (Fig. 3A) compared to that of the control but was less intense compared to the staining with anti-SCRs 1 to 4. Binding of factor H and FHL-1 was dose dependent, as upon incubation in increasing concentrations of NHS-EDTA (5, 40, and 80%) the intensity of the signal increased accordingly (Fig. 3B).
FIG. 3.
Determination by FACS of complement regulators surface bound to C. albicans. (A) Cells were incubated in NHS-EDTA, washed, and incubated with an antiserum recognizing both factor H and FHL-1 (anti-SCRs 1 to 4) or with an antiserum specific for SCRs 19 and 20 of factor H (anti-SCRs 19 and 20). Cells incubated in buffer instead of EDTA-NHS were used as a control. (B) Dose-dependent deposition of factor H and FHL-1 to intact C. albicans. Cells were incubated in buffer containing 5, 40, or 80% NHS-EDTA. After washing, cells were incubated with an antiserum recognizing both factor H and FHL-1 (anti-SCRs 1 to 4). Cells incubated in buffer were used as a control.
Binding of purified factor H and FHL-1 to intact C. albicans cells.
To further confirm, quantitate, and test the specificity of binding, binding assays were repeated with purified factor H and purified recombinant FHL-1, both labeled with 125I. After incubation with the labeled proteins the unbound protein was removed by centrifugation. The fraction of surface bound protein is expressed as a percentage and is compared to the total radioactivity used. Binding was prominent at a cell concentration of 109 and was directly dependent on the available surface area. At a low cell concentration less binding was seen (data not shown).
The specificity of binding was further assayed by competition experiments, which confirmed the specific effect of this interaction. Increasing concentrations of unlabeled factor H, ranging from 0.01 to 1 μg per reaction, showed a dose-dependent inhibition to the binding of radiolabeled factor H to C. albicans (Fig. 4). As a control we also measured binding of another serum protein (C3b) and bovine serum albumin to the cells of C. albicans and detected no binding.
FIG. 4.
Binding of radiolabeled factor H or FHL-1 to intact cells of C. albicans. Specificity of binding was confirmed by using 109 cells in direct binding assays. The cells were incubated with either 125I-labeled factor H, indicated concentrations of unlabeled factor H, or unrelated labeled proteins: i.e., C3b or BSA. As a control, radiolabeled protein was incubated in the absence of cells.
Interaction with host complement occurs at 37°C. We therefore also assayed the effect of temperature and compared binding of the immune regulators at 28 and 37°C. Comparable amounts of radiolabeled proteins were bound at 28 and 37°C; however, slightly lower binding values were observed at 37°C (Table 1). Again binding of unrelated proteins C3b and bovine serum albumin was at background levels.
TABLE 1.
Effect of temperature on binding of host immune regulatorsa
| Protein | % of protein bound at:
|
|
|---|---|---|
| 28°C | 37°C | |
| Factor H | 100 | 95 |
| FHL-1 | 70 | 65 |
| C3b | 15 | 13 |
| BSA | 12 | 14 |
Effect of temperature on binding of factor H, FHL-1, C3b, and BSA to C. albicans grown at 28 C or at 37°C. The total amounts of bound proteins are expressed as a percentage compared to binding of factor H to the cell form at 28°C (100%). Binding intensities of the indicated radiolabeled proteins were compared to the values obtained for factor H binding to the cell form at 28°C.
Specificity of the interaction was also assayed with other Candida strains. In addition to C. albicans the other analyzed strains C. glabrata, C. krusei, C. tropicalis, and C. parapsilosis showed similar binding of human host immune regulators and related amounts/concentration of proteins were absorbed to the yeast surface (data not shown).
The effect of heparin to factor H binding.
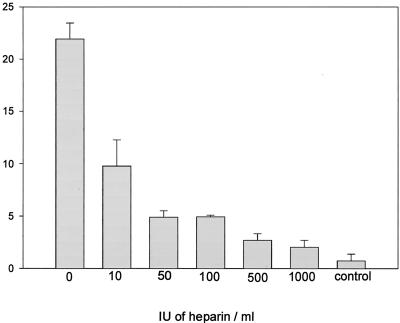
Factor H has heparin interaction sites which have been localized to SCR 7, SCR 13, and SCR 20 (45). As similar domains of factor H are involved in attachment to microbial surfaces, we assayed whether heparin directly affects surface deposition of factor H to C. albicans. Binding of 125I-labeled factor H was assayed in the presence of increasing amounts of heparin. Heparin inhibited attachment of factor H in a dose-dependent manner, and at a concentration of heparin of 1,000 IU/ml, binding of factor H to C. albicans was almost completely abolished (Fig. 5). This indicates that the binding site of factor H to C. albicans overlaps substantially with the heparin binding sites on the native protein or may even be identical.
FIG. 5.
Heparin interferes with binding of factor H to C. albicans. C. albicans cells (109) were incubated with 125I-labeled factor H (approximately 20,000 cpm) in the absence and presence of increasing amount of heparin (10 to 1,000 IU/ml). Bound protein was separated from free radiolabeled protein by centrifugation through a sucrose solution, and the radioactivity of the samples was determined. Results are expressed as a percentage of cell-bound versus free protein. As a control radiolabeled protein was incubated in the absence of heparin or cells.
Absorption and binding of FHL-1 to C. albicans.
As both complement regulators, factor H and FHL-1, are present in NHS and since there is no specific antibody for the detection of FHL-1, we used recombinant FHL-1 to confirm the binding of this protein. C. albicans cells were incubated with FHL-1, and after washing the bound protein was eluted. The wash and elution fractions were separated by SDS-PAGE and analyzed by Western blotting using a goat antiserum against factor H. Recombinant FHL-1 became absorbed by the cells (Fig. 6A, lane 6), as the 42-kDa FHL-1 protein was present in the elution fraction and not in the wash fraction.
FIG. 6.
Localization of the binding region in factor H and FHL-1. C. albicans cells (109) were incubated with recombinant FHL-1 (lanes 5 and 6) and the indicated deletion mutants of FHL-1 and factor H. Both wash (W) and elution (E) fractions were separated by SDS-PAGE and analyzed by Western blotting using goat anti-factor H antiserum detecting factor H, FHL-1, and deletions mutants.
Localization of the binding sites in factor H and FHL-1.
To localize the binding domain(s) on factor H and FHL-1, recombinant deletion mutants of the proteins encompassing SCRs 1 to 5, SCRs 1 to 6, SCRs 8 to 20, SCRs 8 to 11, SCRs 15 to 18, SCRs 15 to 20, and SCRs 19 and 20 were used. The fungal cells were incubated with these mutants, and after washing bound proteins were eluted. The wash and elution fractions were separated by SDS-PAGE and analyzed by Western blotting using the polyclonal goat anti-factor H antiserum. This approach identified two distinct binding domains. One domain is located within SCRs 6 and 7, as binding of FHL-1 and SCRs 1 to 6 (Fig. 6A, lanes 4 and 6) but no binding of SCRs 1 to 5 to C. albicans cells was detected (Fig. 6A, lane 2).
The second binding site, unique to factor H, was localized to SCRs 19 and 20. Recombinant deletion mutants SCRs 8 to 20, SCRs 15 to 20, and SCRs 19 and 20 (Fig. 6A, lane 8, and 6B, lanes 2 and 6) bound to the C. albicans cells while mutant proteins SCRs 8 to 11 and SCRs 15 to 18 (Fig. 6B, lanes 4 and 8) did not bind. In summary, two binding sites in complement regulators factor H and FHL-1 for C. albicans were identified. One site is located in SCRs 6 and 7 and is common to factor H and FHL-1, and the second site is located in the C terminus (i.e., SCRs 19 and 20) of factor H.
Cofactor activity of the cell-bound factor H and FHL-1.
To evaluate whether the surface-attached complement proteins maintain their regulatory function the cofactor activities of factor H and FHL-1 bound to C. albicans were assayed. C. albicans cells were incubated in NHS-EDTA or purified factor H. After intensive washing, purified factor I and 125I-labeled C3b were added, cells were further incubated, and the cell pellet was separated from supernatants by centrifugation. The cleavage of C3b was assayed by SDS-PAGE and autoradiography. Cofactor activity of the bound regulators is judged by disappearance of C3b α′-chain and the appearance of cleavage products of 68 and 46 or 43 kDa (Fig. 7, lanes 1 and 2 and 4 and 5). Cleavage is prominent in the pellet fraction, thus indicating that a significant fraction of the serum regulators is attached and remains bound to the surface. This effect is specific as incubation of Candida in the absence of NHS showed no cofactor activity (Fig. 7, lanes 3 and 6). The results showed significant cofactor activity in the pelleted samples and much less activity in the supernatant fractions. Thus, these experiments confirm that the regulators acquired from human serum remain attached to the surface of candida and in this surface-attached configuration maintain their complement regulatory activity.
FIG. 7.
Cofactor activity of surface-bound factor H and FHL-1. C. albicans cells were preincubated in NHS treated with EDTA (NHS-EDTA), factor H (fH), or buffer (control), and after washing factor I and 125I-labeled C3b were added. Samples from pellet (lanes 1 to 3) and supernatant (lanes 4 to 8) fractions were separated by SDS-PAGE, and C3b and its cleavage products were visualized by autoradiography. Inactivation of C3b to iC3b is observed by the disappearance of the α′-chain of C3b and appearance of α-chain fragments of 68, 46, and 43 kDa. Lanes 3 and 7 represent control reactions in which cells were incubated in buffer. A positive control (+) in which 125I-C3b was incubated in the presence of factors H and I to obtain total cleavage of the α′-chain is shown in lane 7. As a negative control (−) 125I-C3b incubated in the absence of factor H and the presence of factor I shows the absence of unspecific protease (lane 8).
DISCUSSION
In this study we report a novel mechanism of immune evasion for C. albicans. The yeast binds the host complement regulators factor H and FHL-1 and thus gains the ability to control complement activation and the deposition of activation products directly on its surface. The binding of the two central fluid phase regulators, factor H and FHL-1, to the surface of C. albicans was demonstrated by serum absorption, by immunofluorescence, and by FACS analyses. Finally, binding was confirmed by direct binding assays with purified, radiolabeled proteins. Two binding domains were identified by the use of recombinant deletion mutants, and these domains were localized to SCRs 6 and 7 and SCRs 19 and 20. In their surface-attached configuration the bound proteins maintained their complement-regulatory functions. Evasion from phagocytosis and from direct cytolytic effects of complement seems beneficial for C. albicans and may result in a prolonged survival in the human host.
C. albicans activates both the alternative and the classical pathway of complement (28). The complement system has an important role in the resistance against disseminated and cutaneous candidiasis, as in guinea pigs lacking a functional complement system C. albicans readily invades the kidneys, and the animals have a high mortality (16). In addition, antibodies protective against candidiasis require efficient complement deposition on the fungal surface (18). C. albicans has been shown to have resistance mechanisms to avoid the effects of the complement system. In particular, secreted extracellular proteases, which cleave C3, decrease the opsonic activity of C3 and the newly generated proteolytic fragments (25). Similarly, receptors for the cleavage products of the central complement component C3 have been identified on C. albicans (6, 17, 19).
Serum incubation experiments and several biochemical approaches confirm binding of factor H and FHL-1 to the surface of C. albicans. Serum used in these experiments was always treated with EDTA to prevent indirect binding of factor H and FHL-1 via C3b molecules deposited on the C. albicans surface. Several independent approaches presented in this work show binding of the human complement regulators factor H and FHL-1 to C. albicans. The various methods reveal different apparent affinities for factor H and FHL-1. The concentration of factor H in plasma is approximately 500 μg/ml, and that of FHL-1 is 50 μg/ml (13, 15). Thus, the concentration of factor H is about 10-fold higher than that of FHL-1, and due to different molecular masses their molar ratio in plasma is about 3.5 to 1. In adsorption experiments preferential binding of factor H is suggested (Fig. 1). However, the quantitative analysis by FACS (Fig. 3) and direct binding assays (Fig. 4B) with purified, radiolabeled proteins indicate different affinities, with FHL-1 representing 67% (FACS) or 28% (binding assay) of the surface bound proteins.
The use of recombinant deletion mutants identified two binding sites on factor H and FHL-1 which, mediate surface attachment to C. albicans. The first domain, shared by factor H and FHL-1, was located within SCRs 6 and 7, and the second site, specific for factor H, was located within SCRs 19 and 20. With these binding sites, regulators are oriented with the C-terminal ends to the yeast surface and the N-terminal complement regulatory regions pointing to the outside. As the complement regulatory domains of both proteins are located in the N-terminal region this type of attachment allows C3 inactivation in direct vicinity of the cell surface.
Several immune receptors, or complement receptor-like proteins, have been reported on C. albicans. Surface molecules with different molecular masses (42, 55, 66, and 130 kDa) have been identified and suggested to bind C3d or iC3b (1, 19). Based on antibody reactivities and requirement for divalent cations a 60-kDa complement receptor 2 (CR2) and a 130-kDa CR3-like protein have been characterized. As mutants of C. albicans with a reduced CR3-like activity are avirulent in animal models, these C3 binding proteins have been considered to be interesting virulence factors (35). The human CR3 receptor (also termed Mac1, CD11b/CD18, or αMβ2), which is expressed on neutrophils, represents a receptor for factor H (9), acts as an adhesion ligand for human neutrophils, and has been reported to play an essential role for leukocyte adhesion to C. albicans. In this regard it is tempting to speculate whether the corresponding C. albicans CR3-like protein has similar properties and may actually represent the ligand for factor H and FHL-1.
Acquisition of host proteins, particularly human complement regulators, seems to represent a central theme in immune evasion of human pathogens (for a review, see references 37 and 46). An increasing number of human pathogens has been shown to bind and acquire soluble human complement regulators from the plasma, such as the AP regulators factor H, FHL-1 and the classical pathway regulator C4BP. Such organisms include S. pyogenes (27), S. pneumoniae (34), B. burgdorferi (2, 21, 29), Yersinia enterocolitica (8), N. gonorrhoeae (39, 40), N. meningitidis (38), E. granulosus (8), and the human immunodeficiency virus (42). For some pathogens the microbial binding proteins responsible for attachment of the regulators have been identified. These include M protein for S. pyogenes (27); complement regulator-acquiring surface protein (CRASP-1), OspE, and CRASPs 2 to 4 of B. burgdorferi (21, 29); the sialylated lipo-oligosaccharide and major outer membrane porin of N. gonorrhoeae (39, 40); the Hic protein of S. pneumoniae (23); filamentous hemagglutinin of B. pertussis (4); and gp120 and gp41 from human immunodeficiency virus (42).
C. albicans is directly accessible to genetic approaches, and wild type as well as mutant and knockout strains can be directly tested by several virulence assays. Therefore, the identification and cloning of C. albicans molecules involved in interaction and binding of human immune regulators is central for future work and is likely to result in the identification of new virulence factors.
Acknowledgments
We thank M. Dahse (Department of Drug Testing, Hans Knoell Institute) for kind and skillful help with the FACS analyses and A. Fuchs for expert technical assistance.
This study was financially supported by the Deutscher Akademischer Austauschdienst (DAAD), the Deutsche Forschungsgemeinschaft, and the Thüringer Ministerium für Wissenschaft, Forschung und Kunst (to P.F.Z.) and by the Österreichischer Forschungsfond (to R.W.).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alaei, S., C. Larcher, C. Ebenbichler, W. M. Prodinger, J. Janatova, and M. P. Dierich. 1993. Isolation and biochemical characterization of the iC3b receptor of Candida albicans.Infect. Immun. 61:1395-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alitalo, A., T. Meri, L. Rämö, T. S. Jokiranta, T. Heikkilä, I. J. Seppälä, J. Oksi, M. Viljanen, and S. Meri. 2001. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. 69:3685-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck-Sague, C., S. Banerjee, and W. R. Jarvis. 1993. Infectious diseases and mortality among US nursing home residents. Am. J. Public Health 83:1739-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borg, M., and R. Ruchel. 1988. Expression of extracellular acid proteinase by proteolytic Candida spp. during experimental infection of oral mucosa. Infect. Immun. 56:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderone, R. A., and P. C. Braun. 1991. Adherence and receptor relationships of Candida albicans. Microbiol. Rev. 55:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderone, R. A., L. Linehan, E. Wadsworth, and A. L. Sandberg. 1988. Identification of C3d receptors on Candida albicans. Infect. Immun. 56:252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutler, J. E. 1991. Putative virulence factors of Candida albicans. Annu. Rev. Microbiol. 45:187-218. [DOI] [PubMed] [Google Scholar]
- 8.Diaz, A., A. Ferreira, and R. B. Sim. 1997. Complement evasion by Echinococcus granulosus: sequestration of host factor H in the hydatid cyst wall. J. Immunol. 158:3779-3786. [PubMed] [Google Scholar]
- 9.DiScipio, R. G., P. J. Daffern, I. U. Schraufstatter, and P. Sriramarao. 1998. Human polymorphonuclear leukocytes adhere to complement factor H through an interaction that involves αMβ2 (CD11b/CD18). J. Immunol. 160:4057-4066. [PubMed] [Google Scholar]
- 10.Edwards, J. E., Jr. 1991. Invasive candida infections: evolution of a fungal pathogen. N. Engl. J. Med. 324:1060-1062. [DOI] [PubMed] [Google Scholar]
- 11.Farries, T. C., T. Seya, R. A. Harrison, and J. P. Atkinson. 1990. Competition for binding sites on C3b by CR1, CR2, MCP, factor B and factor H. Complement. Inflamm. 7:30-41. [DOI] [PubMed] [Google Scholar]
- 12.Fisher-Hoch, S. P., and L. Hutwagner. 1995. Opportunistic candidiasis: an epidemic of the 1980s. Clin. Infect Dis. 21:897-904. [DOI] [PubMed] [Google Scholar]
- 13.Fontaine, M., M. J. Demares, V. Koistinen, A. J. Day, C. Davrinche, R. B. Sim, and J. Ripoche. 1989. Truncated forms of human complement factor H. Biochem. J. 258:927-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friese, M. A., J. Hellwage, T. S. Jokiranta, S. Meri, H. H. Peter, H. Eibel, and P. F. Zipfel. 1999. FHL-1/reconectin and factor H: two human complement regulators which are encoded by the same gene are differently expressed and regulated. Mol. Immunol. 36:809-818. [DOI] [PubMed] [Google Scholar]
- 16.Gelfand, J. A., D. L. Hurley, A. S. Fauci, and M. M. Frank. 1978. Role of complement in host defense against experimental disseminated candidiasis. J. Infect. Dis. 138:9-16. [DOI] [PubMed] [Google Scholar]
- 17.Gilmore, B. J., E. M. Retsinas, J. S. Lorenz, and M. K. Hostetter. 1988. An iC3b receptor on Candida albicans: structure, function, and correlates for pathogenicity. J. Infect. Dis. 157:38-46. [DOI] [PubMed] [Google Scholar]
- 18.Han, Y., T. R. Kozel, M. X. Zhang, R. S. MacGill, M. C. Carroll, and J. E. Cutler. 2001. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J. Immunol. 167:1550-1557. [DOI] [PubMed] [Google Scholar]
- 19.Heidenreich, F., and M. P. Dierich. 1985. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect. Immun. 50:598-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellwage, J., T. S. Jokiranta, V. Koistinen, O. Vaarala, S. Meri, and P. F. Zipfel. 1999. Functional properties of complement factor H-related proteins FHR-3 and FHR-4: binding to the C3d region of C3b and differential regulation by heparin. FEBS. Lett. 462:345-352. [DOI] [PubMed] [Google Scholar]
- 21.Hellwage, J., T. Meri, T. Heikkilä, A. Alitalo, J. Panelius, P. Lahdenne, I. J. Seppälä, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 22.Horstmann, R. D., H. J. Sievertsen, J. Knobloch, and V. A. Fischetti. 1988. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. USA 85:1657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janulczyk, R., F. Iannelli, A. G. Sjöholm, G. Pozzi, and L. Bjorck. 2000. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 275:37257-37263. [DOI] [PubMed] [Google Scholar]
- 24.Johnsson, E., K. Berggard, H. Kotarsky, J. Hellwage, P. F. Zipfel, U. Sjöbring, and G. Lindahl. 1998. Role of the hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J. Immunol. 161:4894-4901. [PubMed] [Google Scholar]
- 25.Kaminishi, H., H. Miyaguchi, T. Tamaki, N. Suenaga, M. Hisamatsu, I. Mihashi, H. Matsumoto, H. Maeda, and Y. Hagihara. 1995. Degradation of humoral host defense by Candida albicans proteinase. Infect. Immun. 63:984-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koistinen, V., S. Wessberg, and J. Leikola. 1989. Common binding region of complement factors B, H and CR1 on C3b revealed by monoclonal anti-C3d. Complement. Inflamm. 6:270-280. [DOI] [PubMed] [Google Scholar]
- 27.Kotarsky, H., J. Hellwage, E. Johnsson, C. Skerka, H. G. Svensson, G. Lindahl, U. Sjöbring, and P. F. Zipfel. 1998. Identification of a domain in human factor H and factor H-like protein-1 required for the interaction with streptococcal M proteins. J. Immunol. 160:3349-3354. [PubMed] [Google Scholar]
- 28.Kozel, T. R. 1996. Activation of the complement system by pathogenic fungi. Clin. Microbiol. Rev. 9:34-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 30.Kühn, S., C. Skerka, and P. F. Zipfel. 1995. Mapping of the complement regulatory domains in the human factor H-like protein 1 and in factor H. J. Immunol. 155:5663-5670. [PubMed] [Google Scholar]
- 31.Kühn, S., and P. F. Zipfel. 1995. The baculovirus expression vector pBSV-8His directs secretion of histidine-tagged proteins. Gene 162:225-229. [DOI] [PubMed] [Google Scholar]
- 32.Kühn, S., and P. F. Zipfel. 1996. Mapping of the domains required for decay acceleration activity of the human factor H-like protein 1 and factor H. Eur. J. Immunol. 26:2383-2387. [DOI] [PubMed] [Google Scholar]
- 33.Misasi, R., H. P. Huemer, W. Schwaeble, E. Solder, C. Larcher, and M. P. Dierich. 1989. Human complement factor H: an additional gene product of 43 kDa isolated from human plasma shows cofactor activity for the cleavage of the third component of complement. Eur. J. Immunol. 19:1765-1768. [DOI] [PubMed] [Google Scholar]
- 34.Neeleman, C., S. P. Geelen, P. C. Aerts, M. R. Daha, T. E. Mollnes, J. J. Roord, G. Posthuma, H. van Dijk, and A. Fleer. 1999. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect. Immun. 67:4517-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ollert, M. W., E. Wadsworth, and R. A. Calderone. 1990. Reduced expression of the functionally active complement receptor for iC3b but not for C3d on an avirulent mutant of Candida albicans. Infect. Immun. 58:909-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pangburn, M. K., R. D. Schreiber, and H. J. Muller-Eberhard. 1977. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein β1H for cleavage of C3b and C4b in solution. J. Exp. Med. 146:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaller, M., and R. Wenzel. 1992. Impact of the changing epidemiology of fungal infections in the 1990s. Eur. J. Clin. Microbiol. Infect. Dis. 11:287-291. [DOI] [PubMed] [Google Scholar]
- 38.Ram, S., F. G. Mackinnon, S. Gulati, D. P. McQuillen, U. Vogel, M. Frosch, C. Elkins, H. K. Guttormsen, L. M. Wetzler, M. Oppermann, M. K. Pangburn, and P. A. Rice. 1999. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol. Immunol. 36:915-928. [DOI] [PubMed] [Google Scholar]
- 39.Ram, S., D. P. McQuillen, S. Gulati, C. Elkins, M. K. Pangburn, and P. A. Rice. 1998. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J. Exp. Med. 188:671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ram, S., A. K. Sharma, S. D. Simpson, S. Gulati, D. P. McQuillen, M. K. Pangburn, and P. A. Rice. 1998. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J. Exp. Med. 187:743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salacinski, P. R., C. McLean, J. E. Sykes, V. V. Clement-Jones, and P. J. Lowry. 1981. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen). Anal. Biochem. 117:136-146. [DOI] [PubMed] [Google Scholar]
- 42.Stoiber, H., A. Clivio, and M. P. Dierich. 1997. Role of complement in HIV infection. Annu. Rev. Immunol. 15:649-674. [DOI] [PubMed] [Google Scholar]
- 43.Weiler, J. M., M. R. Daha, K. F. Austen, and D. T. Fearon. 1976. Control of the amplification convertase of complement by the plasma protein β1H. Proc. Natl. Acad. Sci. USA 73:3268-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, M. X., and T. R. Kozel. 1998. Mannan-specific immunoglobulin G antibodies in normal human serum accelerate binding of C3 to Candida albicans via the alternative complement pathway. Infect. Immun. 66:4845-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zipfel, P. F., J. Hellwage, M. A. Friese, G. Hegasy, S. T. Jokiranta, and S. Meri. 1999. Factor H and disease: a complement regulator affects vital body functions. Mol. Immunol. 36:241-248. [DOI] [PubMed] [Google Scholar]
- 46.Zipfel, P. F., and C. Skerka. 1999. FHL-1/reconectin: a human complement and immune regulator with cell-adhesive function. Immunol. Today 20:135-140. [DOI] [PubMed] [Google Scholar]