Abstract
A new strain of Babesia microti (KR-1) was isolated from a Connecticut resident with babesiosis by hamster inoculation and adapted to C3H/HeJ and BALB/c mice. To examine the relative importance of humoral and cellular immunity for the control of B. microti infection, we compared the course of disease in wild-type BALB/c mice with that in BALB/c SCID mice, JHD-null (B-cell-deficient) mice, and T-cell receptor αβ (TCRβ−/−) or gamma interferon (IFN-γ) (IFN-γ−/−) knockout mice following inoculation with the KR-1-strain. SCID mice and TCRαβ knockouts sustained a severe but nonlethal parasitemia averaging 35 to 45% infected erythrocytes. IFN-γ-deficient mice developed a less severe parasitemia but were able to clear the infection. In contrast, in six of eight JHD-null mice, the levels of parasitemia were indistinguishable from those in the wild-type animals. These data indicate that cellular immunity is critical for the clearance of B. microti in BALB/c mice but that disease resolution can occur even in the absence of IFN-γ.
The genus Babesia is defined by more than 100 species that collectively infect a wide spectrum of mammals throughout much of the world (11, 14). Maintenance of Babesia parasites in nature requires competent vertebrate and nonvertebrate hosts. In the northeastern United States, Babesia microti cycles between the white-footed field mouse (Peromyscus leucopus) and the deer tick (Ixodes scapularis) (11). Most human cases of babesiosis originate from B. microti sporozoites that are injected into the bloodstream by infected I. scapularis nymphs during feeding. However, some cases have originated from tainted blood transfusions (7). Humans infected with B. microti may be asymptomatic carriers, experience flu-like symptoms, or occasionally suffer fatal complications (11, 15).
The widespread recognition of babesiosis as an emerging zoonotic disease (11, 14) has spurred improvements in drug therapies (16) and diagnostics (17, 19, 22), as well as interest in the development of a babesiosis vaccine (11). A better understanding of the immune response to babesial infection could be helpful in designing a safe and efficacious vaccine. While previous reports on mice have shown a strong role for T cells and the Th1 cytokine gamma interferon (IFN-γ) in the control of B. microti infection (6, 13, 21), results regarding the role of humoral immunity in parasite clearance have been less clear-cut (2, 4, 13). In this study, we mouse adapted a freshly obtained B. microti clinical isolate in order to clarify the contributions of cellular and humoral immunity to disease resolution in a murine model.
Our strain was isolated from a 42-year-old asplenic male afflicted with grade 4 lymphoma who lived in northern Connecticut and worked in an area along the Connecticut coastline where Babesia is endemic. He reported a tick bite approximately 1 week prior to the onset of illness. Giemsa stains of the patient's blood at the time of presentation revealed a parasitemia of approximately 5%. Indirect immunofluorescence assay (using the GI strain as antigen) revealed serum immunoglobulin M and G titers of 128 and >1,024, respectively. PCR analysis of whole blood using the Bab1/Bab4 primer pair specific for the B. microti 18S rRNA gene (19) yielded a product of the appropriate size (238 bp).
To isolate the strain, subsequently designated KR-1, 1 ml of blood (containing approximately 2 × 108 infected erythrocytes) obtained prior to therapy was injected into a 3-week-old female golden Syrian hamster (Mesocricetus auratus). Golden Syrian hamsters are naturally susceptible to infection with B. microti and have been used to confirm suspected cases in humans (9). The inoculated hamster was monitored daily by Giemsa staining of tail blood samples. Shortly after the parasitemia peaked at about 1%, the hamster was anesthetized and its blood was harvested by cardiac puncture. Following three additional passages, parasitemias of >30% were obtained. Because golden Syrian hamsters are maintained as outbred colonies, they are not well suited for analysis of the immune processes induced during babesial infection. Consequently, we next adapted the strain to C3H/HeJ mice, which are highly susceptible to infection with B. microti (1, 20), by injecting 1 ml of whole hamster blood (approximately 108 infected erythrocytes) intraperitoneally into 4- to 6-week-old female C3H/HeJ mice. As before, parasitemia was monitored by PCR analysis and Giemsa staining of tail blood samples. By the sixth passage in C3H/HeJ mice, parasitemias reached 6%.
Although most human babesial infections in the United States are due to B. microti, in recent years, infections due to piroplasms other than B. microti have been reported (14). To characterize the KR-1 isolate, we determined the nucleotide sequence of the Bab1/Bab4 18S rRNA amplicon. The sequence was identical to those of the GI and Ruebush-Peabody strains, both of which were isolated from residents of Nantucket Island, Mass. It was also identical to a sequence obtained from B. microti-infested Ixodes ricinus ticks from Slovenia (8). The latter finding is in accord with a report stating that allopatric differentiation of B. microti strains by the 18S rRNA gene sequence is low (23). In contrast to rRNAs, proteins under immune pressure may reveal higher-resolution phylogenies among strains and/or serve as ecological and pathogenic markers. Indeed, this has been demonstrated by using an immunogenic protein family (BMN1 to -6) identified by serological screening of a B. microti expression library (12). The primer pair used for phylogenetic analysis in that study, however, yielded amplicons from only 50% of the samples known to be B. microti positive and did not yield a product with our strain.
While the BALB/c background is considered moderately permissive for B. microti (1), preliminary experiments revealed relatively low levels of parasitemia following inoculation with 0.5 ml of blood (108 infected erythrocytes) from a parasitemic C3H/HeJ mouse. Such low levels of parasitemia, however, were considered to be advantageous for comparison of the course of disease in wild-type animals and animals with various immune deficiencies. Consequently, in a series of experiments, we compared the course of infection in wild-type BALB/c mice with that in BALB/c SCID mice, JHD-null (B-cell-deficient) mice (3), T-cell receptor αβ knockout (TCRβ−/−) mice, and IFN-γ knockout (IFN-γ−/−) mice. Wild-type, SCID, TCRβ−/−, and IFN-γ−/− mice were purchased as breeding pairs from The Jackson Laboratory (Bar Harbor, Maine) and maintained at the University of Connecticut Health Center by one of us (T.V.R.). A JHD-null breeding pair was the generous gift of Mark Shlomchik (Yale University).
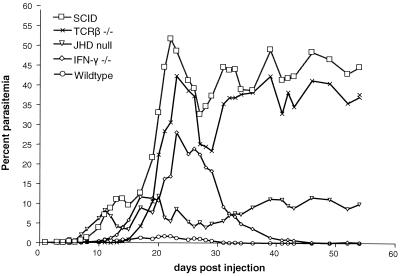
Figure 1 presents the parasitemia curves for a comprehensive experiment in which all five groups (each composed of 6-week-old male mice, six mice per group) were compared head to head following inoculation with 200 μl of phosphate-buffered saline-diluted blood (106 parasitized red cells) from a heavily parasitemic BALB/c SCID mouse. The P values for days 10, 20, 23, 31, 41, and 54 are presented in Table 1. Parasitemia levels in the wild-type controls remained relatively low, peaking at approximately 2% on or about day 20 postinjection. In contrast, SCID mice developed a severe chronic parasitemia that fluctuated around 40%. TCRαβ-deficient mice yielded a parasitemia curve indistinguishable from that of SCID mice; this is consistent with previous studies that have demonstrated a critical role for T cells, particularly CD4+ T cells, in clearing B. microti in mice (6, 13, 21). IFN-γ knockout mice consistently developed parasitemias less severe than those of SCID mice yet significantly greater than those of wild-type controls. Interestingly, however, unlike their TCRαβ-deficient counterparts, IFN-γ knockout mice were eventually able to resolve their infections. This result differs from a previous study that reported an inability of IFN-γ-deficient BALB/c mice to clear the Munich strain of B. microti (13). Differences in IFN-γ production have been shown in mice infected with different Babesia species (10). Variation in IFN-γ expression also may occur in mice infected with different substrains of B. microti (13, 18). In CBA mice, IFN-γ expression has been shown to increase in the early stages of B. microti (King strain) infection (5). Our data indicate that IFN-γ is important for control of the early stages of infection in BALB/c mice but that disease resolution will occur despite a defective Th1 response.
FIG. 1.
Parasitemia curves following inoculation of BALB/c wild-type, SCID, TCRβ−/−, IFN-γ−/−, and JHD-null (B-cell-deficient) mice with 106 erythrocytes infected with the KR-1 strain of B. microti. Each point represents the average parasitemia of six mice determined from Giemsa-stained tail blood samples. P values between experimental groups at selected time points are shown in Table 1.
TABLE 1.
P valuesa for parasitemia curves at selected time points postinoculation
| Comparison |
P value
|
|||||
|---|---|---|---|---|---|---|
| Day 10 | Day 20 | Day 23 | Day 31 | Day 41 | Day 54 | |
| SCID vs T Δb | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
| SCID vs B Δc | >0.05 | >0.05 | <0.001 | <0.001 | <0.001 | <0.001 |
| SCID vs IFN-γ Δd | >0.05 | >0.05 | <0.05 | <0.001 | <0.001 | <0.001 |
| SCID vs +/+e | >0.05 | <0.05 | <0.001 | <0.001 | <0.001 | <0.001 |
| TΔ vs BΔ | >0.05 | >0.05 | <0.001 | <0.001 | <0.01 | <0.001 |
| TΔ vs IFN-γ Δ | >0.05 | >0.05 | >0.05 | <0.001 | <0.001 | <0.001 |
| TΔ vs +/+ | >0.05 | >0.05 | <0.001 | <0.001 | <0.001 | <0.001 |
| BΔ vs IFN-γΔ | >0.05 | >0.05 | <0.05 | >0.05 | >0.05 | >0.05 |
| BΔ vs +/+ | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
| IFN-γ Δ vs +/+ | >0.05 | >0.05 | <0.001 | >0.05 | >0.05 | >0.05 |
P values were derived by one-way analysis of variance and Tukey's multiple-comparison test. Significant P values (< 0.05) are in bold.
TΔ, TCRβ−/− mice.
BΔ, JHD null mice.
IFN-γΔ, IFN-γ−/− mice.
+/+, wild-type BALB/c mice.
The parasitemia curve for JHD-null mice was not significantly different from that of wild-type controls. However, the JHD mutants displayed two distinct phenotypes. Four mice developed transient parasitemias that quickly resolved to background levels, whereas two mice experienced delayed parasitemias that rose to a chronic level of approximately 30%. In a separate experiment, two additional JHD-null mice developed only transient parasitemias. To ensure that all of the JHD-null mice whose results are depicted in Fig. 1 were deficient in mature B cells, we examined circulating lymphocyte populations by flow cytometry with a panleukocyte panel consisting of goat anti-mouse anti-CD3-fluorescein isothiocyanate, anti-CD19-phycoerythrin-Cy5, and anti-CD45R (eBioscience, San Diego, Calif.). All six JHD-null mice displayed significant reductions in mature B-cell markers in comparison with wild-type controls without considerable variation among staining populations (data not shown).
A dichotomy exists in the literature concerning the role of humoral immunity in resolving B. microti infection. Cavacini et al. (2) showed that antibody-depleted BALB/c mice were as resistant as wild-type mice to the King strain of B. microti. Additionally, Igarashi et al. (13) were unable to passively protect BALB/c mice with convalescent-phase sera from animals infected with B. microti (Munich strain) prior to a homologous challenge. However, Chen et al. (4) demonstrated that convalescent-phase immune serum can inhibit B. microti growth in short-term in vitro growth assays. Our results also suggest that antibody is relatively unimportant in the BALB/c background. Because genetic variation among mice can influence B. microti-host interactions considerably (1), the role for humoral immunity in the resolution of babesiosis needs to be examined in other genetic backgrounds. Moreover, the finding that humoral immunity is unnecessary for clearance of an established infection does not necessarily preclude a role for antibodies in protection against tick transmission of B. microti sporozoites, the pre-erythrocytic form of the parasite that is likely to display antibody-accessible antigens. The mouse-adapted KR-1 strain used for the needle inoculation studies reported herein is infectious throughout the tick-mouse cycle (unpublished observations) and, thus, can be used to assess this notion.
Acknowledgments
We thank Pat Tomas, Diane Christianson, and Kathy Freeman for superb technical assistance and Karsten Hazlett for many helpful suggestions.
This study was funded through Connecticut Innovations Critical Technology grant 99CT018 (J.D.R. and P.J.K.); grants AI-29735 (J.D.R.), AI-39075 (S.K.W.), and AI-50228 (T.V.R.) from the National Institute of Allergy and Infectious Diseases; and cooperative agreement U50CCU119575 from the Centers for Disease Control and Prevention, U.S. Public Health Service (S.K.W.).
Editor: A. D. O'Brien
REFERENCES
- 1.Aguilar-Delfin, I., M. J. Homer, P. J. Wettstein, and D. H. Persing. 2001. Innate resistance to Babesia infection is influenced by genetic background and gender. Infect. Immun. 69:7955-7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavacini, L. A., L. A. Parke, and W. P. Weidanz. 1990. Resolution of acute malarial infections by T cell-dependent non-antibody-mediated mechanisms of immunity. Infect. Immun. 58:2946-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan, O. T., M. P. Madaio, and M. J. Shlomchik. 1999. B cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunity. J. Immunol. 163:3592-3596. [PubMed] [Google Scholar]
- 4.Chen, D., D. B. Copeman, J. Burnell, and G. W. Hutchinson. 2000. Helper T cell and antibody responses to infection of CBA mice with Babesia microti. Parasite Immunol. 22:81-88. [DOI] [PubMed] [Google Scholar]
- 5.Chen, D., D. B. Copeman, G. W. Hutchinson, and J. Burnell. 2000. Inhibition of growth of cultured Babesia microti by serum and macrophages in the presence or absence of T cells. Parasitol. Int. 48:223-231. [DOI] [PubMed] [Google Scholar]
- 6.Clark, I. A., and A. C. Allison. 1974. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature 252:328-329. [DOI] [PubMed] [Google Scholar]
- 7.Dobroszcki, J., B. L. Herwaldt, F. Boctor, J. R. Miller, J. Linden, M. L. Eberhard, J. J. Yoon, N. M. Ali, H. B. Tanowitz, F. Graham, L. M. Weiss, and M. Wittner. 1999. A cluster of transfusion-associated babesiosis cases traced to a single asymptomatic donor. JAMA 281:927-930. [DOI] [PubMed] [Google Scholar]
- 8.Duh, D., M. Petrovec, and T. Avsic-Zupanc. 2001. Diversity of Babesia infecting European sheep ticks (Ixodes ricinus). J. Clin. Microbiol. 39:3395-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etkind, P., J. Piesman, T. K. Ruebush, A. Spielman, and D. D. Juranek. 1980. Methods for detecting Babesia microti infection in wild rodents. J. Parasitol. 66:107-110. [PubMed] [Google Scholar]
- 10.Hemmer, R. M., D. A. Ferrick, and P. A. Conrad. 2000. Role of T cells and cytokines in fatal and resolving experimental babesiosis: protection in TNFRp55−/− mice infected with the human Babesia WA1 parasite. J. Parasitol. 86:736-742. [DOI] [PubMed] [Google Scholar]
- 11.Homer, M. J., I. Aguilar-Delfin, S. R. Telford III, P. J. Krause, and D. H. Persing. 2000. Babesiosis. Clin. Microbiol. Rev. 13:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homer, M. J., E. S. Bruinsma, M. J. Lodes, M. H. Moro, S. Telford III, P. J. Krause, L. D. Reynolds, R. Mohamath, D. R. Benson, R. L. Houghton, S. G. Reed, and D. H. Persing. 2000. A polymorphic multigene family encoding an immunodominant protein from Babesia microti. J. Clin. Microbiol. 38:362-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igarashi, I., R. Suzuki, S. Waki, Y. Tagawa, S. Seng, S. Tum, Y. Omata, A. Saito, H. Nagasawa, Y. Iwakura, N. Suzuki, T. Mikami, and Y. Toyoda. 1999. Roles of CD4+ T cells and gamma interferon in protective immunity against Babesia microti infection in mice. Infect. Immun. 67:4143-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kjemtrup, A. M., and P. A. Conrad. 2001. Emerging perspectives on human babesiosis, p. 175-195. In W. M. Scheld, W. A. Craig, and J. M. Hughes (ed.), Emerging infections. ASM Press, Washington, D.C.
- 15.Krause, P. J. 2002. Babesiosis. Med. Clin. N. Am. 86:361-374. [DOI] [PubMed] [Google Scholar]
- 16.Krause, P. J., T. Lepore, V. K. Sikand, J. Gadbaw, Jr., G. Burke, S. R. Telford III, P. Brassard, D. Pearl, J. Azlanzadeh, D. Christianson, D. McGrath, and A. Spielman. 2000. Atovaquone and azithromycin for the treatment of babesiosis. N. Engl. J. Med. 343:1454-1458. [DOI] [PubMed] [Google Scholar]
- 17.Lodes, M. J., R. L. Houghton, E. S. Bruinsma, R. Mohamath, L. D. Reynolds, D. R. Benson, P. J. Krause, S. G. Reed, and D. H. Persing. 2000. Serological expression cloning of novel immunoreactive antigens of Babesia microti. Infect. Immun. 68:2783-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsubara, J., M. Koura, and T. Kamiyama. 1993. Infection of immunodeficient mice with a mouse-adapted substrain of the gray strain of Babesia microti. J. Parasitol. 79:783-786. [PubMed] [Google Scholar]
- 19.Persing, D. H., D. Mathiesen, W. F. Marshall, S. R. Telford, A. Spielman, J. W. Thomford, and P. A. Conrad. 1992. Detection of Babesia microti by polymerase chain reaction. J. Clin. Microbiol. 30:2097-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruebush, M. J., and W. L. Hanson. 1979. Susceptibility of five strains of mice to Babesia microti of human origin. J. Parasitol. 65:430-433. [PubMed] [Google Scholar]
- 21.Ruebush, M. J., and W. L. Hanson. 1980. Thymus dependence of resistance to infection with Babesia microti of human origin in mice. Am. J. Trop. Med. Hyg. 29:507-515. [DOI] [PubMed] [Google Scholar]
- 22.Ryan, R., P. J. Krause, J. Radolf, K. Freeman, A. Spielman, R. Lenz, and A. Levin. 2001. Diagnosis of babesiosis using an immunoblot serologic test. Clin. Diagn. Lab Immunol. 8:1177-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuji, M., Q. Wei, A. Zamoto, C. Morita, S. Arai, T. Shiota, M. Fujimagari, A. Itagaki, H. Fujita, and C. Ishihara. 2001. Human babesiosis in Japan: epizootiologic survey of rodent reservoir and isolation of new type of Babesia microti-like parasite. J. Clin. Microbiol. 39:4316-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]