Abstract
1. Rapid and slow changes in the absorbance of isolated frog retinae produced by exposure to brief flashes were studied at temperatures between 5 and 30 °C.
2. Rapid changes observed at 475 nm consist of a transient increase of absorbance followed by an exponential decay to a new level of absorbance which is lower than before the flash exposure.
3. The new level of absorbance determines the initial conditions of slow changes following the rapid ones. At higher temperatures, the loss of absorbance during the rapid changes is greater than at lower temperatures. Accordingly, the slow reactions start at lower levels of absorbance when the temperature is high.
4. Quantitative analysis showed that the rapid reactions can be described in terms of two consecutive reactions followed by an equilibrium reaction: the light-controlled formation of lumirhodopsin, decay of lumirhodopsin to metarhodopsin I, and the equilibrium reaction between the metarhodopsins I and II.
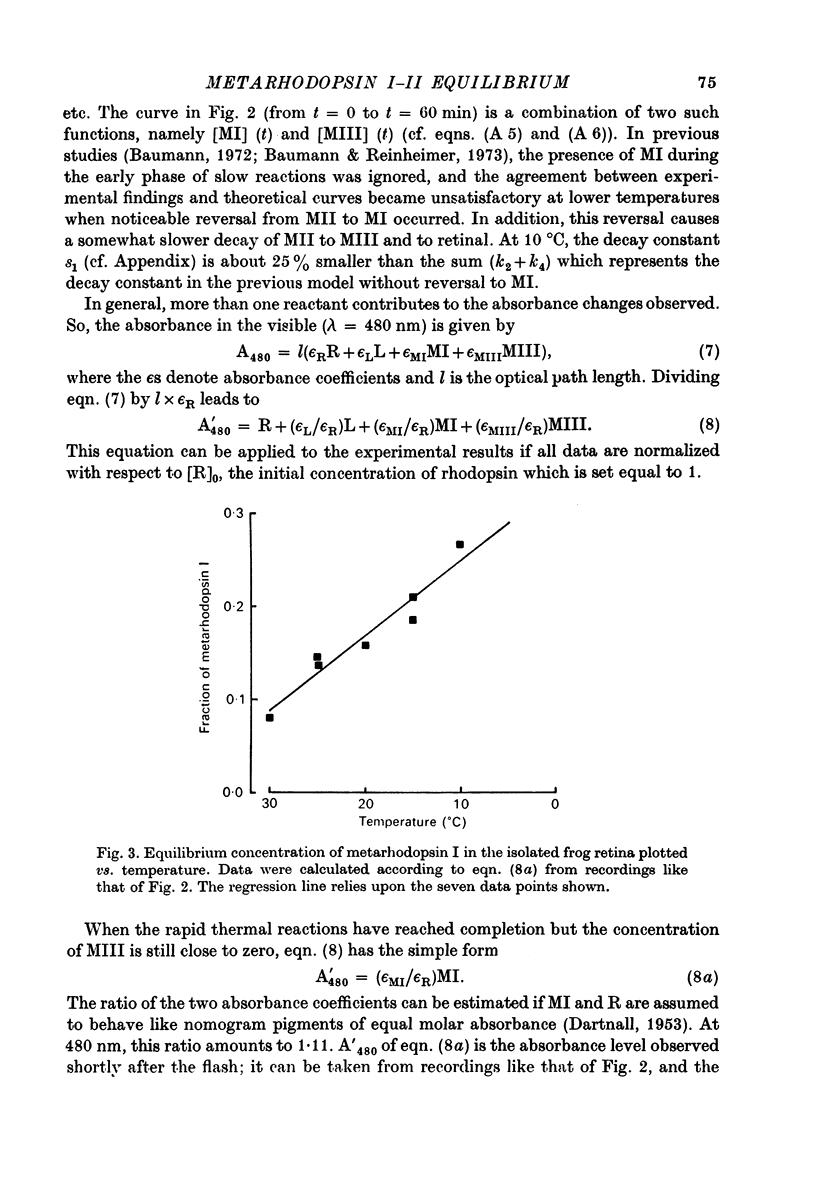
5. The slow absorbance changes observed in the visible (λ = 480 nm) are due to metarhodopsin I and to metarhodopsin III. Metarhodopsin I decays during the early phase of slow reactions but can noticeably influence the kinetics at lower temperatures.
6. The activation energy of the lumirhodopsin decay is 22.5 kcal/mole, that of the conversion of metarhodopsin I into metarhodopsin II is 30.1 kcal/mole. The entropy change associated with the metarhodopsin I-II equilibrium amounts to +34 cal/mole. K.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann C. Flash photolysis of rhodopsin in the isolated frog retina. Vision Res. 1970 Sep;10(9):789–798. doi: 10.1016/0042-6989(70)90158-6. [DOI] [PubMed] [Google Scholar]
- Baumann C. Kinetics of slow thermal reactions during the bleaching of rhodopsin in the perfused frog retina. J Physiol. 1972 May;222(3):643–663. doi: 10.1113/jphysiol.1972.sp009819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C. The formation of metarhodospin380 in the retinal rods of the frog. J Physiol. 1976 Jul;259(2):357–366. doi: 10.1113/jphysiol.1976.sp011470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARTNALL H. J. A. The interpretation of spectral sensitivity curves. Br Med Bull. 1953;9(1):24–30. doi: 10.1093/oxfordjournals.bmb.a074302. [DOI] [PubMed] [Google Scholar]
- Erhardt F., Ostroy S. E., Abrahamson E. W. Protein configuration changes in the photolysis of rhodopsin. I. The thermal decay of cattle lumirhodopsin in vitro. Biochim Biophys Acta. 1966 Feb 7;112(2):256–264. doi: 10.1016/0926-6585(66)90325-6. [DOI] [PubMed] [Google Scholar]
- Hubbard R., Bownds D., Yoshizawa T. The chemistry of visual photoreception. Cold Spring Harb Symp Quant Biol. 1965;30:301–315. doi: 10.1101/sqb.1965.030.01.032. [DOI] [PubMed] [Google Scholar]
- MATTHEWS R. G., HUBBARD R., BROWN P. K., WALD G. TAUTOMERIC FORMS OF METARHODOPSIN. J Gen Physiol. 1963 Nov;47:215–240. doi: 10.1085/jgp.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroy S. E., Erhardt F., Abrahamson E. W. Protein configuration changes in the photolysis of rhodopsin. II. The sequence of intermediates in thermal decay of cattle metarhodopsin in vitro. Biochim Biophys Acta. 1966 Feb 7;112(2):265–277. doi: 10.1016/0926-6585(66)90326-8. [DOI] [PubMed] [Google Scholar]


