Abstract
1. Extracellular recordings were obtained from spinal dorsal horn cells in acutely spinalized cats anaesthetized with sodium pentobarbitone. The dorsal horn cells studied responded to ipsilateral tactile stimulation of the central pad of the hind foot. Eleven short latency dorsal horn cells driven by electrical stimulation of the foot pad were studied intensively; these short latency dorsal horn cells all discharged within 1.5 msec of the arrival of an afferent volley at the dorsal root entry zone. Electrode tip sites were histologically verified to lie near the medial border of the dorsal horn in the seventh lumbar segment, in Rexed's laminae III and IV. 2. Electrical stimulation of the foot pad not only activated the dorsal horn cells studied, but also produced a reflex discharge which was monitored by recording from the ipsilateral first sacral ventral root, which had been sectioned intradurally and mounted on bipolar recording electrodes. Repeated stimulation of the foot pad at moderate intensities and frequencies (e.g. three times threshold for the ventral root response at 5 Hz) typically produced a transitory increase in the magnitude of the reflex discharge (sensitization) followed by a marked waning of the reflex magnitude (habituation). Within a few minutes following cessation of stimulation, the reflex magnitude returned to its prestimulation value. During repeated bouts of five hundred stimuli each, at frequencies from 1.0 to 10.0 Hz, and intensities 1.5-10.0 times reflex threshold, the firing pattern of a short latency dorsal horn cell was monitored along with the magnitude of the ventral root response. Changes in response patterns of the dorsal horn cells were compared to those of the reflex discharges. 3. The short latency dorsal horn cells fell into two distinct patterns of response. The firing pattern of six dorsal horn cells paralleled the response pattern of the reflex discharge; when the reflex increased in magnitude, each of these dorsal horn cells increased in number of responses per stimulus; when the reflex discharge decreased in magnitude, each of these dorsal horn cells decreased the number of responses per stimulus. These dorsal horn cells were characterized by the following: intermediate thresholds to tactile stimulation, comparable to that of the reflex discharge itself; relatively low numbers of responses per stimulus (mean: 1.3/stimulus); low spontaneous activity rates (once per 10 sec or less). 4. The firing patterns of the other class of short latency dorsal horn cells did not parallel the response pattern of the reflex discharge; these showed only a rather rapid, though moderate, decrease in responses per stimulus over the entire range of intensities and frequencies tested. These five dorsal horn cells were characterized by the following: thresholds of tactile stimulation considerably below that of the reflex discharge itself; bursts of responses following each stimulation (mean: 7...
Full text
PDF





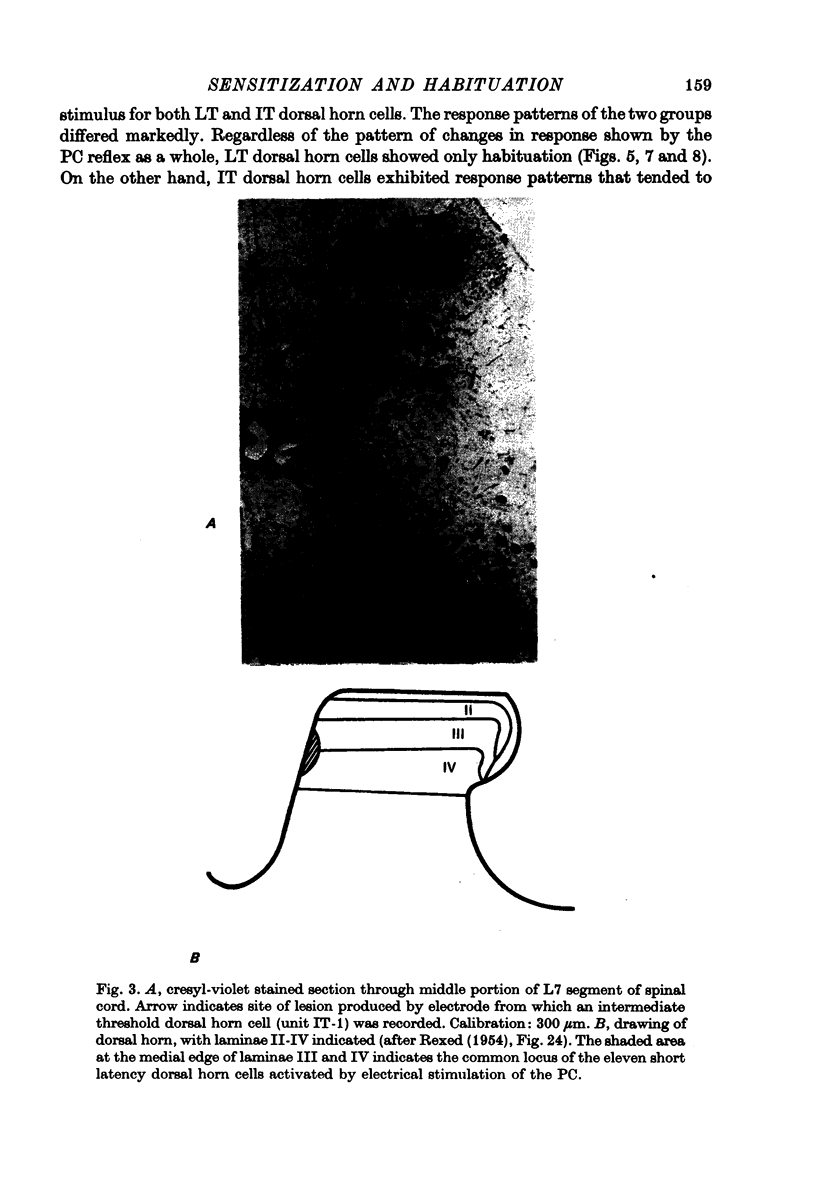







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMETT C. J., GRAY J. A., PALMER J. F. A group of neurones in the dorsal horn associated with cutaneous mechanoreceptors. J Physiol. 1961 May;156:611–622. doi: 10.1113/jphysiol.1961.sp006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. S., Pentreath V. W. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976 Mar 19;105(1):1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Callec J. J., Guillet J. C., Pichon Y., Boistel J. Further studies on synaptic transmission in insects. II. Relations between sensory information and its synaptic integration at the level of a single giant axon in the cockroach. J Exp Biol. 1971 Aug;55(1):123–149. doi: 10.1242/jeb.55.1.123. [DOI] [PubMed] [Google Scholar]
- Capek R., Esplin B. Homosynaptic depression and transmitter turnover in spinal monosynaptic pathway. J Neurophysiol. 1977 Jan;40(1):95–105. doi: 10.1152/jn.1977.40.1.95. [DOI] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V., Kandel E. R. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science. 1976 Dec 10;194(4270):1176–1178. doi: 10.1126/science.11560. [DOI] [PubMed] [Google Scholar]
- Egger M. D., Bishop J. W., Cone C. H. Sensitization and habituation of the plantar cushion reflex in cats. Brain Res. 1976 Feb 20;103(2):215–228. doi: 10.1016/0006-8993(76)90795-2. [DOI] [PubMed] [Google Scholar]
- Egger M. D., Wall P. D. The plantar cushion reflex circuit: an oligosynaptic cutaneous reflex. J Physiol. 1971 Jul;216(2):483–501. doi: 10.1113/jphysiol.1971.sp009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farel P. B. Dual processes control response habituation across a single synapse. Brain Res. 1974 Jun 7;72(2):323–327. doi: 10.1016/0006-8993(74)90874-9. [DOI] [PubMed] [Google Scholar]
- Farel P. B., Glanzman D. L., Thompson R. F. Habituation of a monosynaptic response in vertebrate central nervous system: lateral column-motoneuron pathway in isolated frog spinal cord. J Neurophysiol. 1973 Nov;36(6):1117–1130. doi: 10.1152/jn.1973.36.6.1117. [DOI] [PubMed] [Google Scholar]
- Farel P. B., Thompson R. F. Habituation of a monosynaptic response in frog spinal cord: evidence for a presynaptic mechanism. J Neurophysiol. 1976 Jul;39(4):661–666. doi: 10.1152/jn.1976.39.4.661. [DOI] [PubMed] [Google Scholar]
- Groves P. M., De Marco R., Thompson R. F. Habituation and sensitization of spinal interneuron activity in acute spinal cat. Brain Res. 1969 Jul;14(2):521–525. doi: 10.1016/0006-8993(69)90129-2. [DOI] [PubMed] [Google Scholar]
- Groves P. M., Thompson R. F. Habituation: a dual-process theory. Psychol Rev. 1970 Sep;77(5):419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Iggo A., Ogawa H. Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat's glabrous skin. J Physiol. 1977 Apr;266(2):275–296. doi: 10.1113/jphysiol.1977.sp011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res. 1968;6(2):100–115. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Brunelli M., Byrne J., Castellucci V. A common presynaptic locus for the synaptic changes underlying short-term habituation and sensitization of the gill-withdrawal reflex in Aplysia. Cold Spring Harb Symp Quant Biol. 1976;40:465–482. doi: 10.1101/sqb.1976.040.01.044. [DOI] [PubMed] [Google Scholar]
- Lynn B. The nature and location of certain phasic mechanoreceptors in the cat's foot. J Physiol. 1969 May;201(3):765–773. doi: 10.1113/jphysiol.1969.sp008786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Thompson R. F., Spencer W. A. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966 Jan;73(1):16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Watt D. G., Stauffer E. K., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. J Neurophysiol. 1976 Nov;39(6):1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Crayfish escape behavior and central synapses. II. Physiological mechanisms underlying behavioral habituation. J Neurophysiol. 1972 Sep;35(5):621–637. doi: 10.1152/jn.1972.35.5.621. [DOI] [PubMed] [Google Scholar]



