Abstract
1. Single and double sucrose-gap methods were used to follow changes in membrane potential and conductance of smooth muscle of sheep carotid arteries. 2. K depolarization induced discharges lasting several seconds in various solutions containing Mn, or Mg, or Ca and procaine, sometimes with no other added cations and with only sulphate or ethanesulphonate as anions. 3. Membrane conductance usually rose substantially above resting level in the early part of these discharges, but fell towards resting level during the later part. 4. Large depolarizing currents caused porportionately less voltage displacement than small currents, and reduced voltage displacements induced by superimposed current pulses, even in Cl and HCO3 free solutions, indicating activation of K conductance by depolarization. 5. When procaine was added, or Ca replaced by Mn or Mg, conductance was lower both at rest and on depolarization, and the increase on depolarization often underwent slow inactivation which it never did in simple Ca containing solutions. 6. The results indicate that a slowly inactivated inward current dependent on Ca, Mn or Mg was largely responsible for the slow discharges, and that procaine, Mn or Mg assisted the discharges by reducing the normal rapid outward-rectifying K conductance and allowing it to inactivate on prolonged depolarization.
Full text
PDF

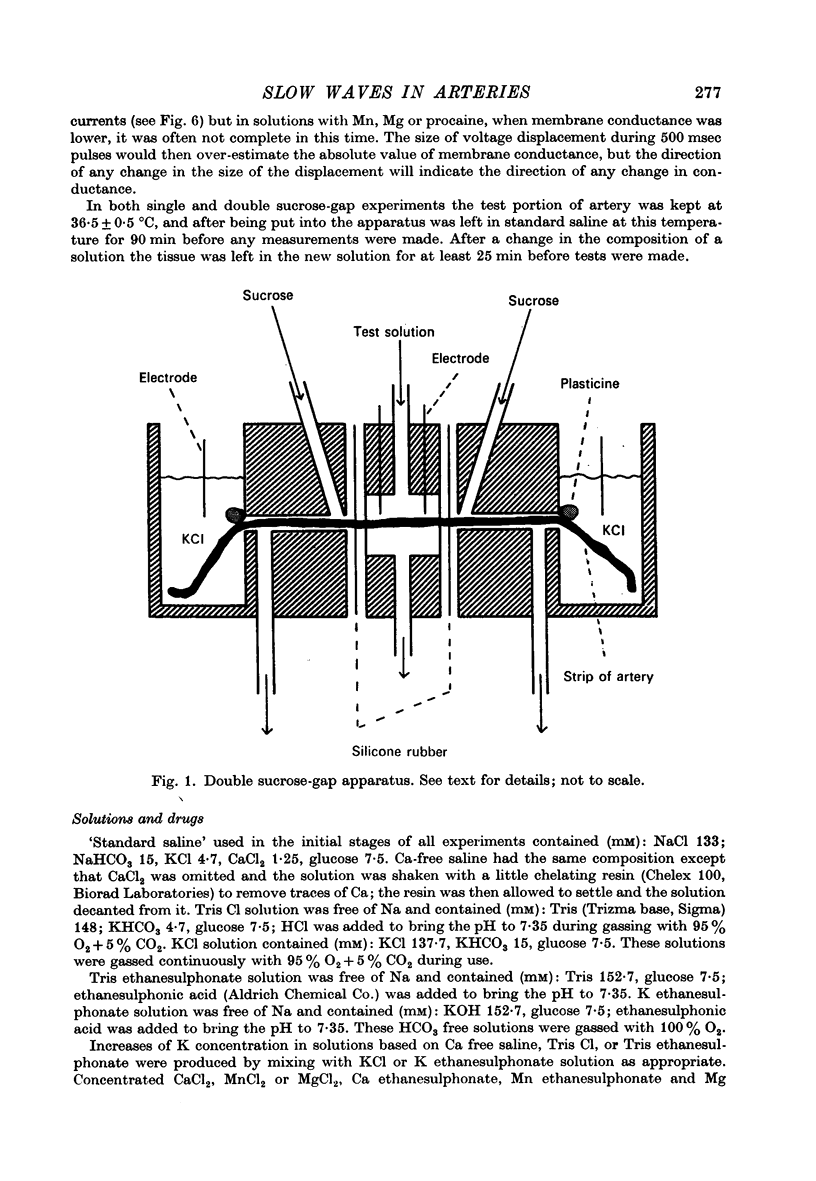

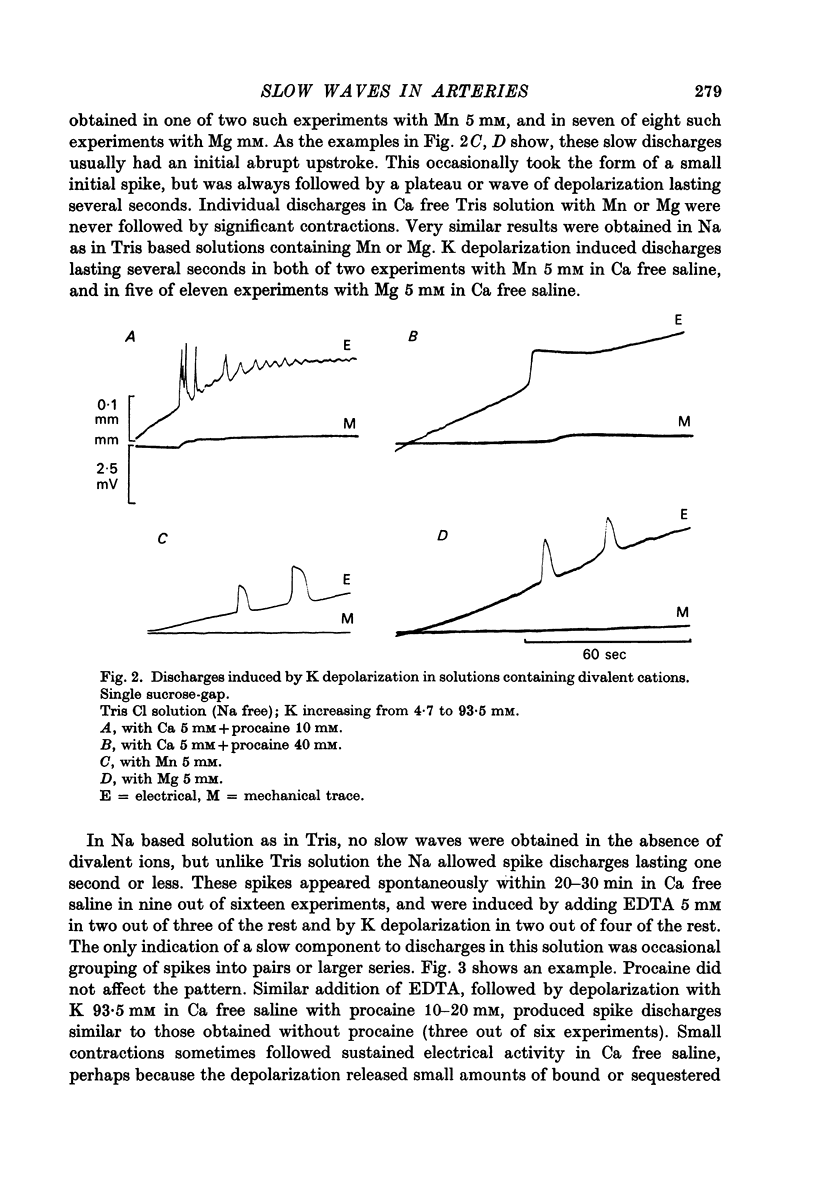
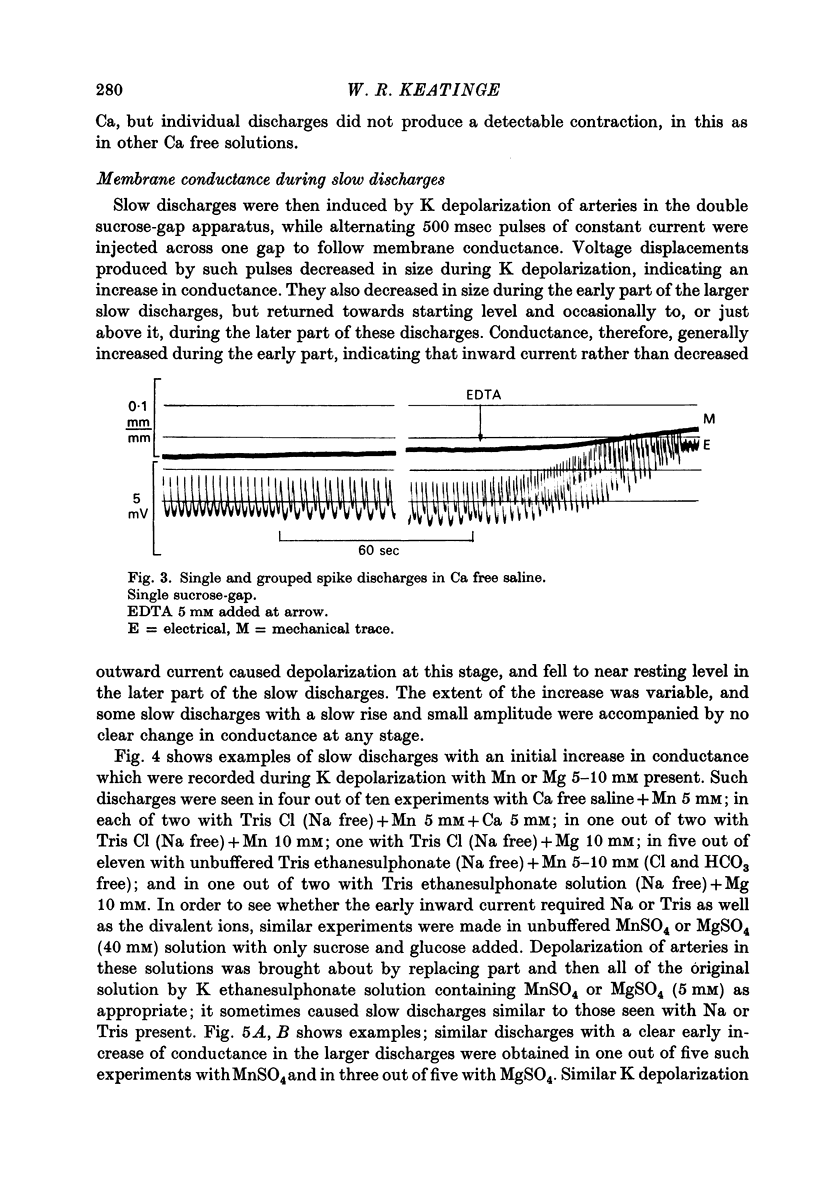
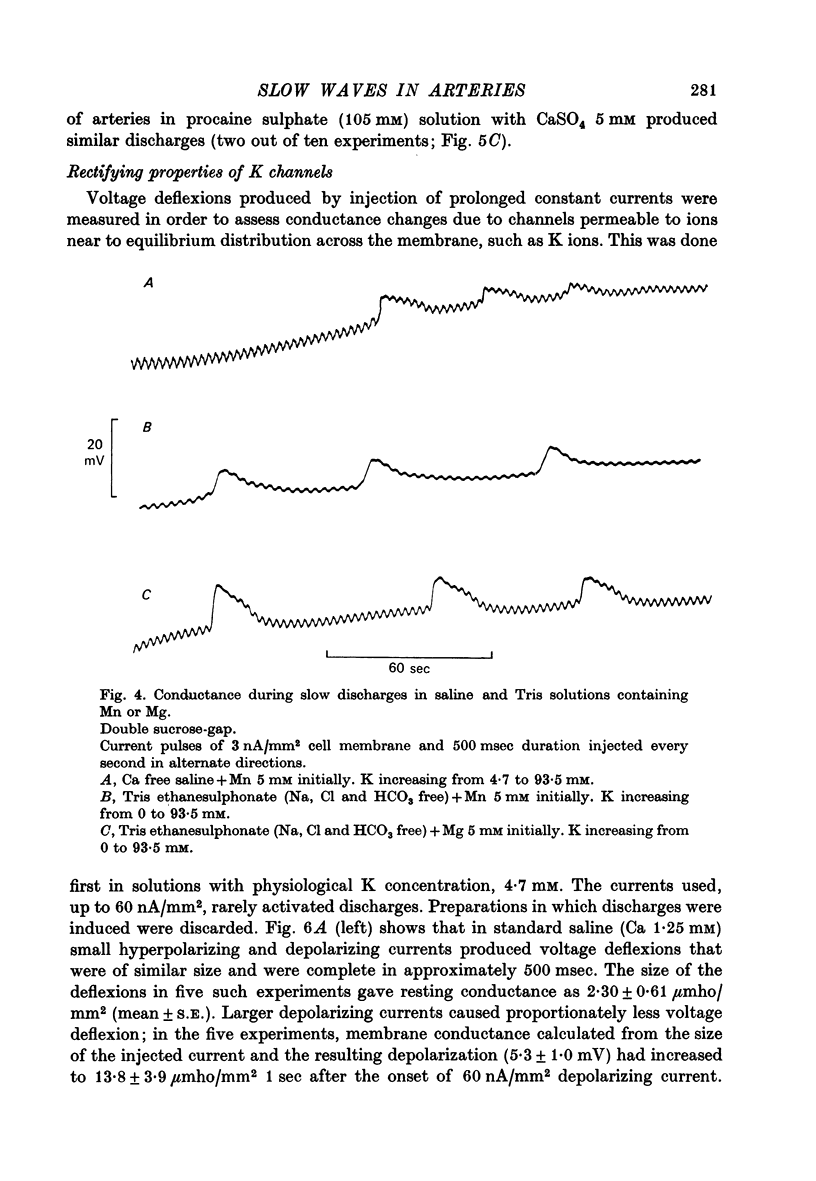




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. C., Jr Voltage-clamp studies on uterine smooth muscle. J Gen Physiol. 1969 Aug;54(2):145–165. doi: 10.1085/jgp.54.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. C., Ramon F., Snyder A. Studies on calcium and sodium in uterine smooth muscle excitation under current-clamp and voltage-clamp conditions. J Gen Physiol. 1971 Sep;58(3):322–339. doi: 10.1085/jgp.58.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASS P., WILEY J. N. ELECTRICAL AND EXTRALUMINAL CONTRACTILE-FORCE ACTIVITY OF THE DUODENUM OF THE DOG. Am J Dig Dis. 1965 Mar;10:183–200. doi: 10.1007/BF02233747. [DOI] [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Effects of stimulating the acetylcholine receptor on the current-voltage relationships of the smooth muscle membrane studied by voltage clamp of potential recorded by micro-electrode. J Physiol. 1975 Aug;250(1):175–202. doi: 10.1113/jphysiol.1975.sp011048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Effects of Ca removal on the smooth muscle of the guinea-pig taenia coli. J Physiol. 1970 Sep;210(1):217–232. doi: 10.1113/jphysiol.1970.sp009205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E., Vereecke J. Adrenaline and the plateau phase of the cardiac action potential. Importance of Ca++, Na+ and K+ conductance. Pflugers Arch. 1969;313(4):300–315. doi: 10.1007/BF00593955. [DOI] [PubMed] [Google Scholar]
- Clusin W. T., Bennett M. V. Calcium-activated conductance in skate electroreceptors: voltage clamp experiments. J Gen Physiol. 1977 Feb;69(2):145–182. doi: 10.1085/jgp.69.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Prosser C. L., Weems W. A. A study of pace-maker activity in intestinal smooth muscle. J Physiol. 1974 Aug;240(3):671–701. doi: 10.1113/jphysiol.1974.sp010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper W. D., Goodford P. J., Hardy C. C., Herring J., Hind C. R., Keatinge W. R. Effect of procaine and tetraethylammonium on 42-K efflux from smooth muscle of sheep carotid arteries. J Physiol. 1975 Mar;246(2):70–71P. [PubMed] [Google Scholar]
- DANIEL E. E. EFFECTS OF INTRA-ARTERIAL PERFUSIONS ON ELECTRICAL ACTIVITY AND ELECTROLYTE CONTENTS OF DOG SMALL INTESTINE. Can J Physiol Pharmacol. 1965 Jul;43:551–577. doi: 10.1139/y65-056. [DOI] [PubMed] [Google Scholar]
- Golenhofen K., Petrányi P. Spikes of smooth muscle in calcium-free solution (isolated taenia coli of the guinea pig). Experientia. 1969 Mar 15;25(3):271–273. doi: 10.1007/BF02034386. [DOI] [PubMed] [Google Scholar]
- Graham J. M., Keatinge W. R. Responses of inner and outer muscle of the sheep carotid artery to injury. J Physiol. 1975 May;247(2):473–482. doi: 10.1113/jphysiol.1975.sp010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivel M. L., Ruckebusch Y. The propagation of segmental contractions along the small intestine. J Physiol. 1972 Dec;227(2):611–625. doi: 10.1113/jphysiol.1972.sp010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN M. E. The effect of changes in sodium chloride concentration on the smooth muscle of the guinea-pig's taenia coli. J Physiol. 1957 May 23;136(3):569–584. doi: 10.1113/jphysiol.1957.sp005782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. Rectifying properties of heart muscle. Nature. 1960 Nov 5;188:495–495. doi: 10.1038/188495a0. [DOI] [PubMed] [Google Scholar]
- Jacobs A., Keatinge W. R. Effects of procaine and lignocaine on electrical and mechanical activity of smooth muscle of sheep carotid arteries. Br J Pharmacol. 1974 Jul;51(3):405–411. doi: 10.1111/j.1476-5381.1974.tb10676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D. D. Ionic basis of intestinal electrical activity. Am J Physiol. 1969 Nov;217(5):1534–1541. doi: 10.1152/ajplegacy.1969.217.5.1534. [DOI] [PubMed] [Google Scholar]
- KEATINGE W. R. MECHANISM OF ADRENERGIC STIMULATION OF MAMMALIAN ARTERIES AND ITS FAILURE AT LOW TEMPERATURES. J Physiol. 1964 Nov;174:184–205. doi: 10.1113/jphysiol.1964.sp007481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., McCullough J. R. Ionic currents in the uterine smooth muscle. J Physiol. 1975 Mar;246(1):1–36. doi: 10.1113/jphysiol.1975.sp010878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Electrical and mechanical response of arteries to stimulation of sympathetic nerves. J Physiol. 1966 Aug;185(3):701–715. doi: 10.1113/jphysiol.1966.sp008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Ionic requirements for arterial action potential. J Physiol. 1968 Jan;194(1):169–182. doi: 10.1113/jphysiol.1968.sp008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Proceedings: Extracellular Ca and response of sheep carotid artery to depolarization. J Physiol. 1976 Jun;258(2):73P–74P. [PubMed] [Google Scholar]
- Keatinge W. R. Sodium flux and electrical activity of arterial smooth muscle. J Physiol. 1968 Jan;194(1):183–200. doi: 10.1113/jphysiol.1968.sp008401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan J. A. Some limitations of the double sucrose gap, and its use in a study of the slow outward current in mammalian ventricular muscle. J Physiol. 1974 Aug;240(3):775–806. doi: 10.1113/jphysiol.1974.sp010634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological studies of the smooth muscle cell membrane of the rabbit common carotid artery. J Gen Physiol. 1971 Jun;57(6):738–751. doi: 10.1085/jgp.57.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F., Keatinge W. R. Electrical behaviour of inner and outer smooth muscle of sheep carotid artery. Nature. 1975 Dec 11;258(5535):534–535. doi: 10.1038/258534a0. [DOI] [PubMed] [Google Scholar]
- Mekata F. Rectification in the smooth muscle cell membrane of rabbit aorta. J Physiol. 1976 Jun;258(2):269–278. doi: 10.1113/jphysiol.1976.sp011419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAJIMA S., IWASAKI S., OBATA K. Delayed rectification and anomalous rectification in frog's skeletal muscle membrane. J Gen Physiol. 1962 Sep;46:97–115. doi: 10.1085/jgp.46.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergerke R., Page S., Talbot M. S. Calcium fluxes in frog heart ventricles. Pflugers Arch. 1969;306(4):357–360. doi: 10.1007/BF00589161. [DOI] [PubMed] [Google Scholar]
- ORKAND R. K., NIEDERGERKE R. HEART ACTION POTENTIAL: DEPENDENCE ON EXTERNAL CALCIUM AND SODIUM IONS. Science. 1964 Nov 27;146(3648):1176–1177. doi: 10.1126/science.146.3648.1176. [DOI] [PubMed] [Google Scholar]
- Ochi R. Manganese-dependent propagated action potentials and their depression by electrical stimulation in guinea-pig myocardium perfused by sodium-free media. J Physiol. 1976 Dec;263(2):139–156. doi: 10.1113/jphysiol.1976.sp011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba M., Sakamoto Y., Tomita T. Effects of sodium, potassium and calcium ions on the slow wave in the circular muscle of the guinea-pig stomach. J Physiol. 1977 May;267(1):167–180. doi: 10.1113/jphysiol.1977.sp011806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Garnier D., Gargouil Y. M., Coraboeuf E. Existence and role of a slow inward current during the frog atrial action potential. Pflugers Arch. 1969;308(2):91–110. doi: 10.1007/BF00587018. [DOI] [PubMed] [Google Scholar]
- Shuba M. F. The effect of sodium-free and potassium-free solutions, ionic current inhibitors and ouabain on electrophysiological properties of smooth muscle of guinea-pig ureter. J Physiol. 1977 Jan;264(3):837–851. doi: 10.1113/jphysiol.1977.sp011697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuba M. F. The mechanism of the excitatory action of catecholamines and histamine on the smooth muscle of guinea-pig ureter. J Physiol. 1977 Jan;264(3):853–864. doi: 10.1113/jphysiol.1977.sp011698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Lerman L. Role of divalent cations in excitation of squid giant axons. Am J Physiol. 1967 Dec;213(6):1465–1474. doi: 10.1152/ajplegacy.1967.213.6.1465. [DOI] [PubMed] [Google Scholar]
- Trautwein W., McDonald T. F., Tripathi O. Calcium conductance and tension in mammalian ventricular muscle. Pflugers Arch. 1975;354(1):55–74. doi: 10.1007/BF00584503. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Effect of current flow on the membrane potential of cardiac muscle. J Physiol. 1951 Oct 29;115(2):227–236. doi: 10.1113/jphysiol.1951.sp004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S. Heart: electrophysiology. Annu Rev Physiol. 1974;36:155–169. doi: 10.1146/annurev.ph.36.030174.001103. [DOI] [PubMed] [Google Scholar]
- Yanaga T., Holland W. C. Effect of manganese on transmembrane potential and contractility of atrial muscle. Am J Physiol. 1969 Nov;217(5):1280–1285. doi: 10.1152/ajplegacy.1969.217.5.1280. [DOI] [PubMed] [Google Scholar]


