Abstract
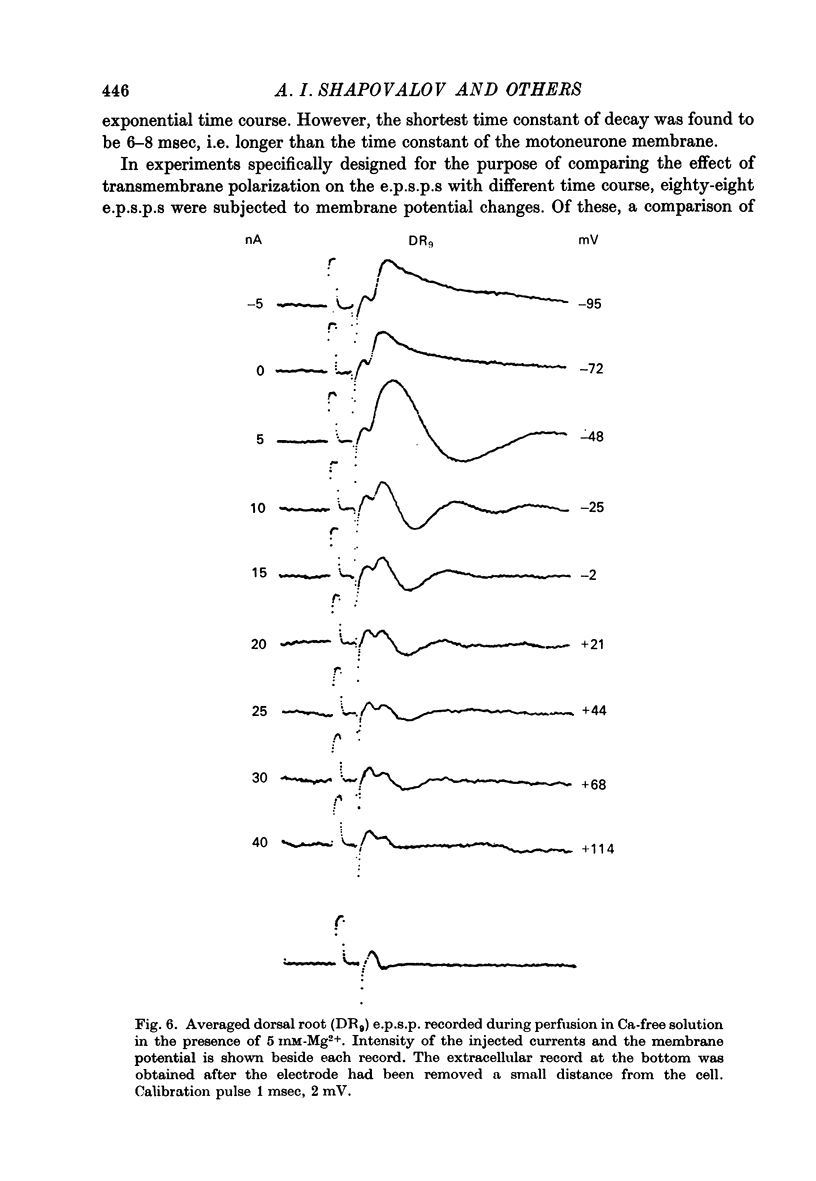
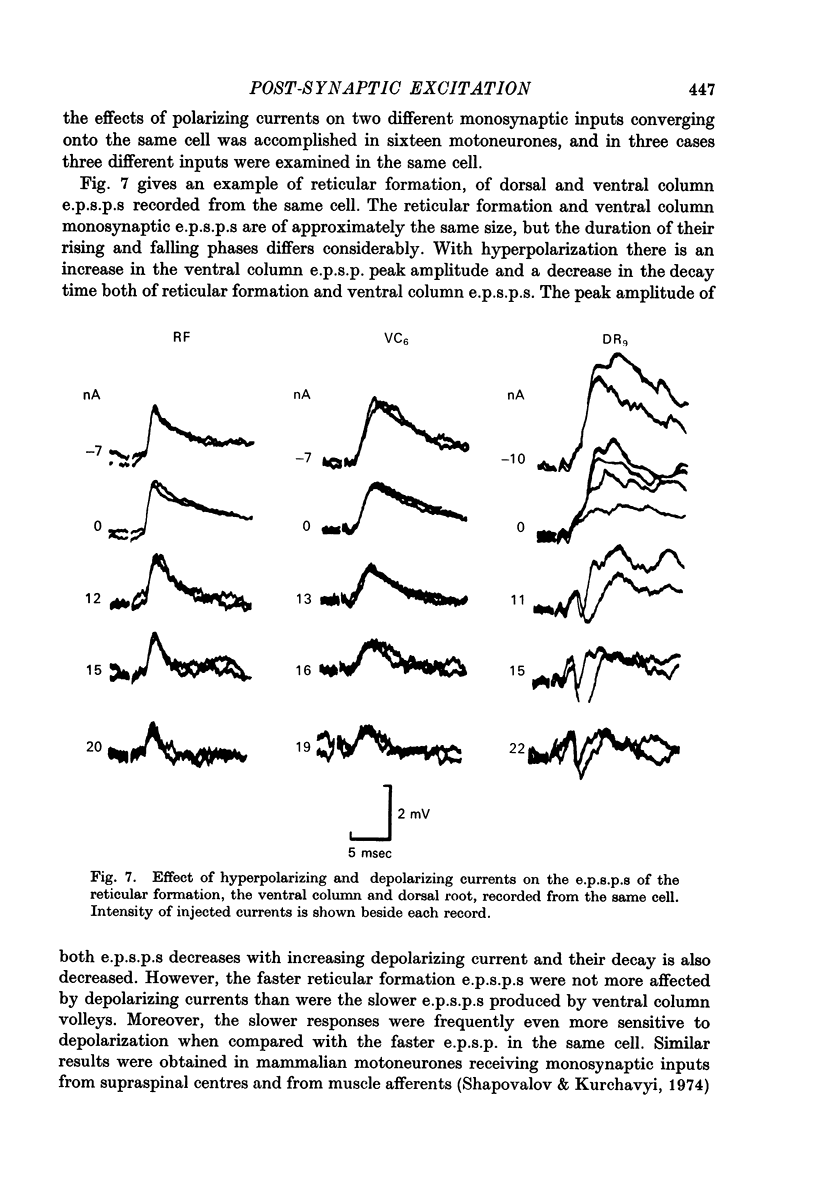
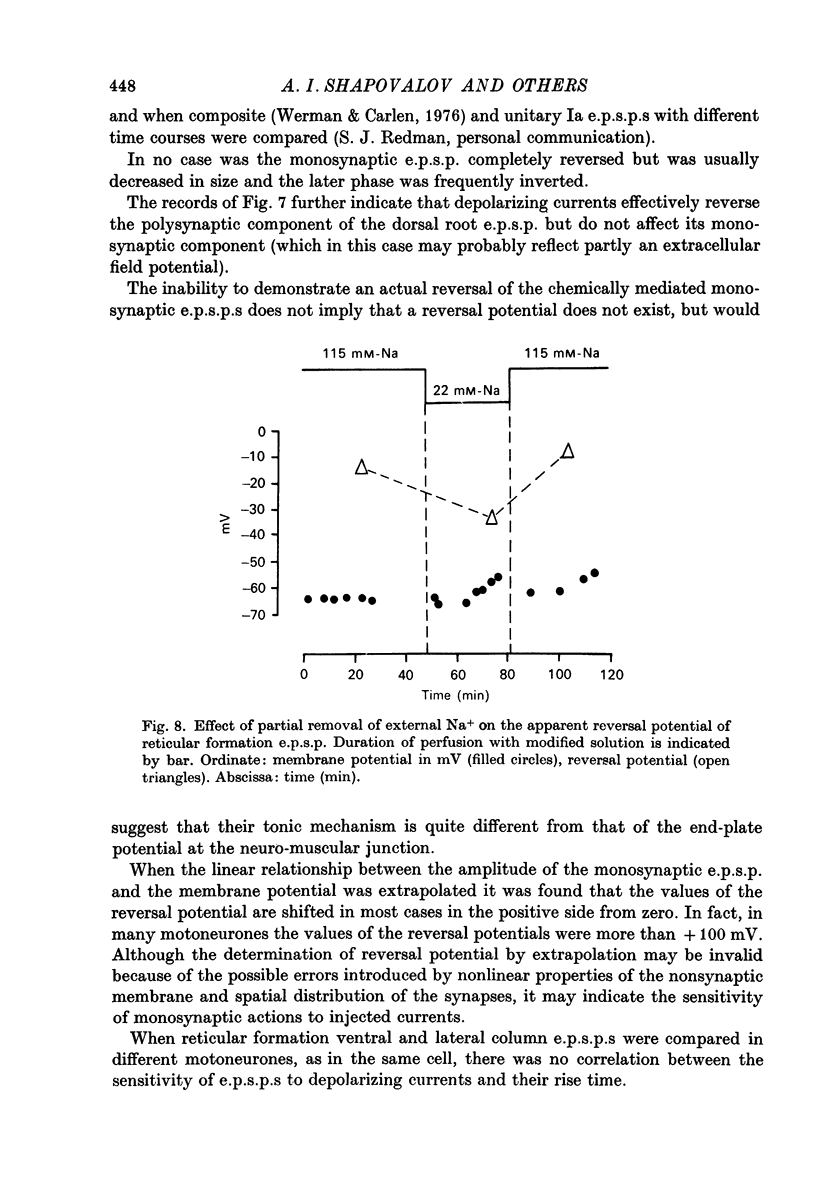
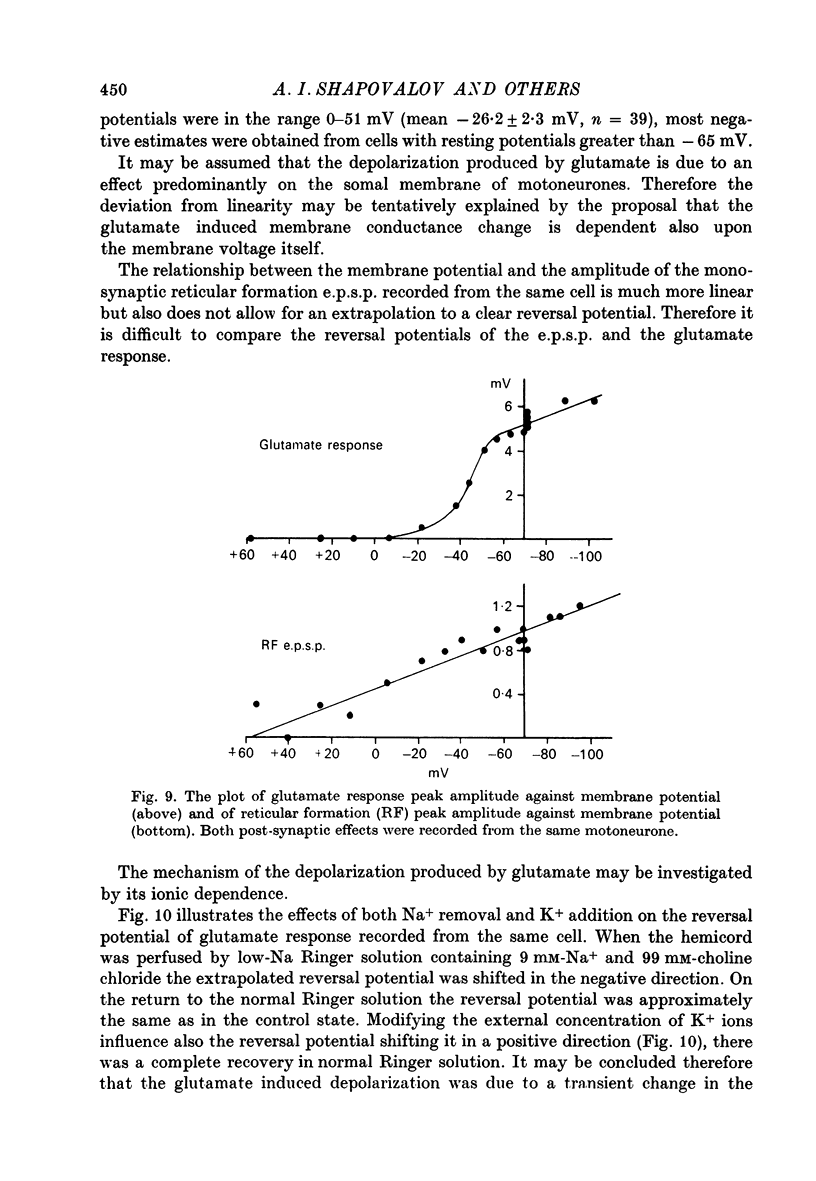
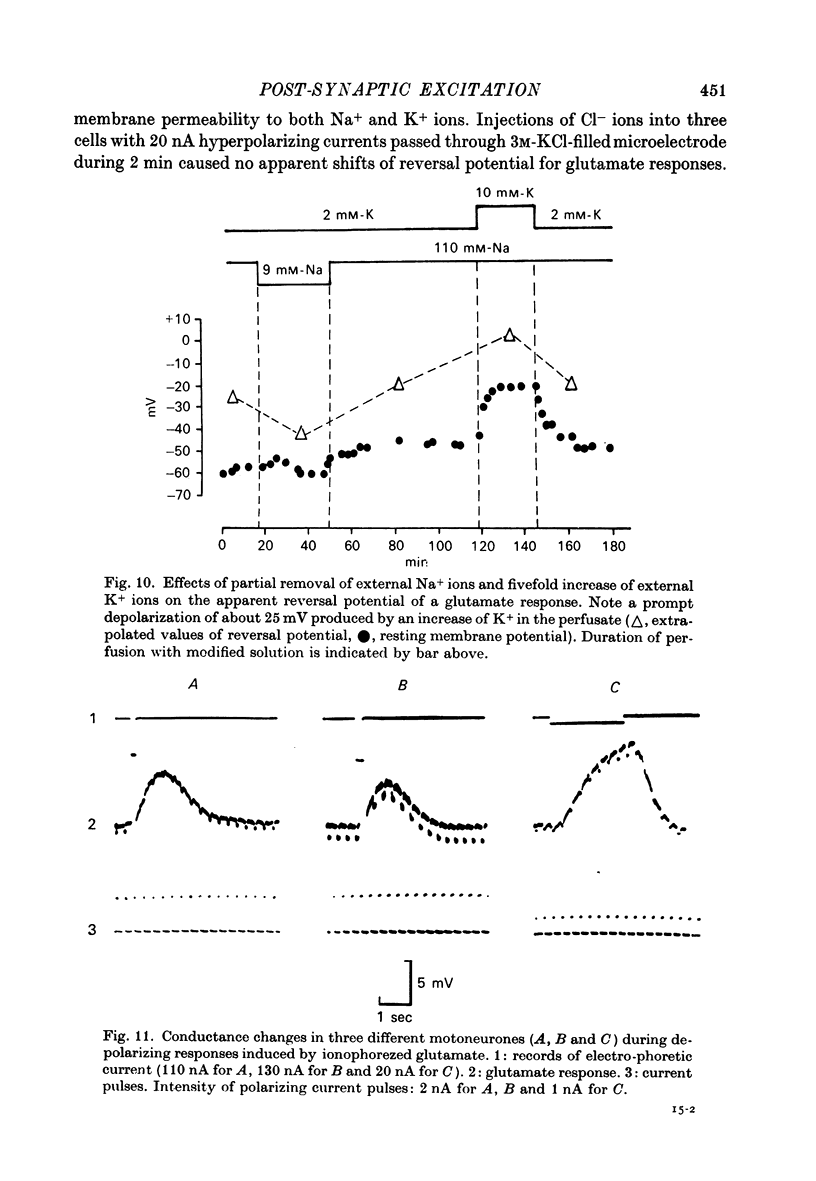
1. Post-synaptic excitation produced in motoneurones of the isolated perfused frog spinal cord by different monosynaptic inputs and by ionophoretically applied glutamate was analysed with intracellular recording technique. 2. Ca2+-deficient, high Mg2+ (5--20 mM) media or addition of Mn2+ (2mM) or Co2+ (5 mM) reversibly abolished chemically mediated e.p.s.p.s derived from medullary reticular formation, ventral and lateral columns, but not the short-latency, rapidly rising e.p.s.p.s derived from dorsal roots or muscle nerves, suggesting electric coupling between some primary afferents and spinal motoneurones. This conclusion is consistent with the dynamic properties of dorsal root e.p.s.p.s, their small sensitivity to cooling, and with results of correction of intracellular records made for contribution of extracellular field potential. E.p.s.p.s evoked by ventral root stimulation were also insensitive to Ca2+-lack and presence of 5--10 mM-Mg2+. 3. As the post-synaptic membrane was made more negative the amplitude of electrotonic dorsal root e.p.s.p.s was increased, and it was decreased by depolarizing currents. No reversal of the early part of the electrotonic e.p.s.p. was observed, although the presence of the local response would account for the occasional reversal of its later phase seen with depolarization. 4. When hyperpolarizing and depolarizing currents were applied to motoneurones in which chemically mediated e.p.s.p.s of the reticular cells, the ventral and lateral columns, were evoked, the actual reversal of the early part of e.p.s.p. was not observed, and there was no correlation between the sensitivity of the e.p.s.p.s to injected currents and their time course. The positive values of the extrapolated reversal potentials and the effects of changes in ionic content of perfusing media suggest that synaptically released transmitter triggers off the Na permeability of the subsynaptic membrane. 5. The amplitude of depolarization produced by ionophoretically applied glutamate depends non-linearly on membrane potential and the curvature of this dependence differs from that seen with chemically mediated s.p.s.p.s. The asymptotic nature of this relationship is explicable by a dependence of the membrane conductance change upon the membrane voltage. 6. The results of conductance measurements during the glutamate induced depolarization, the values of apparent reversal potentials and their dependence on external Na+ and K+ and internal Cl- is explicable by the opening post-synaptic channel gates for Na+ and closing post-synaptic channel gates for K+. 7. Chemical and electrical transmission in the amphibian cord is discussed in relation to recent anatomical findings.
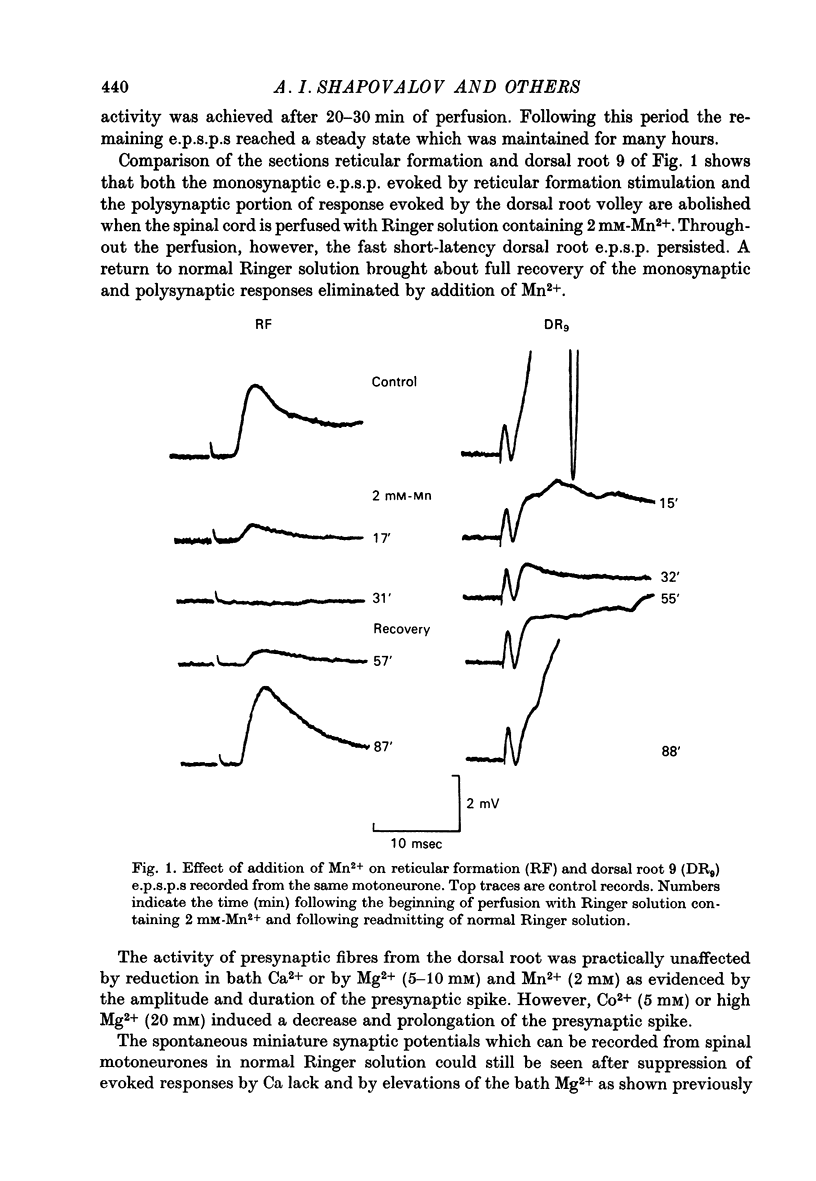
Full text
PDF


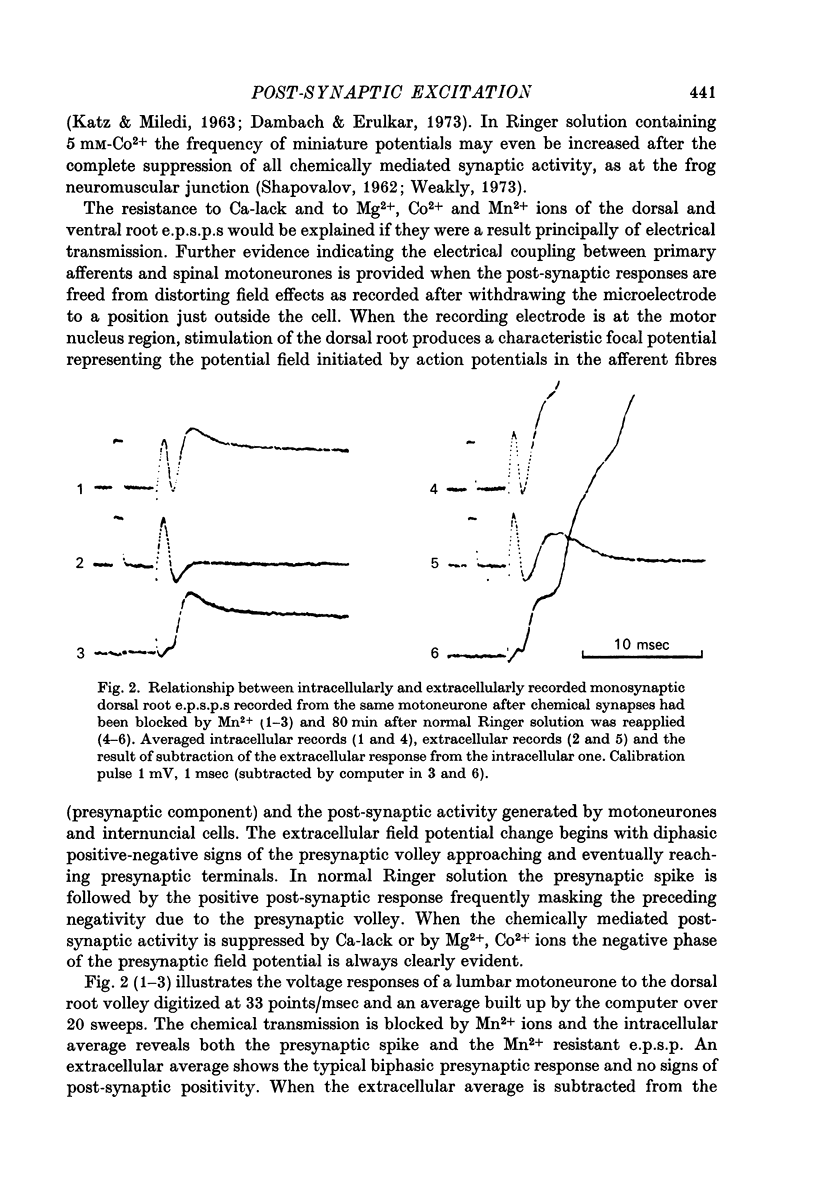

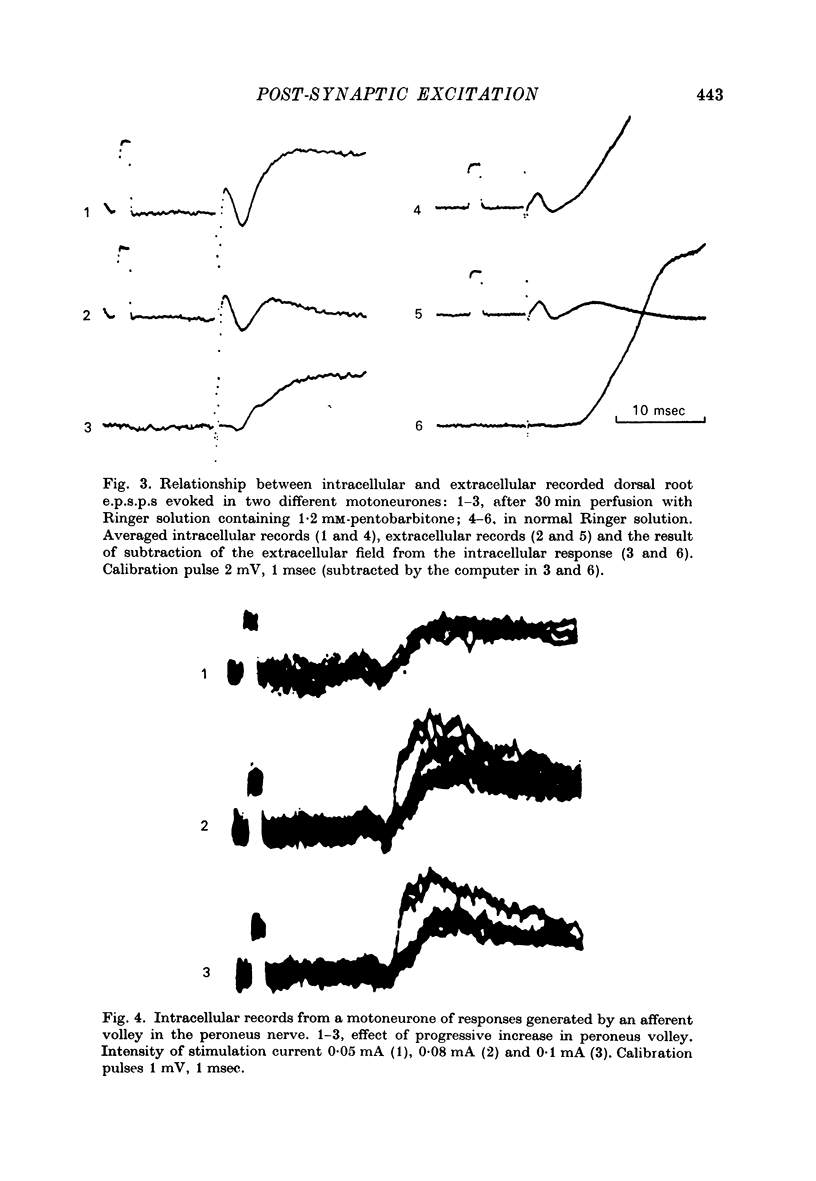
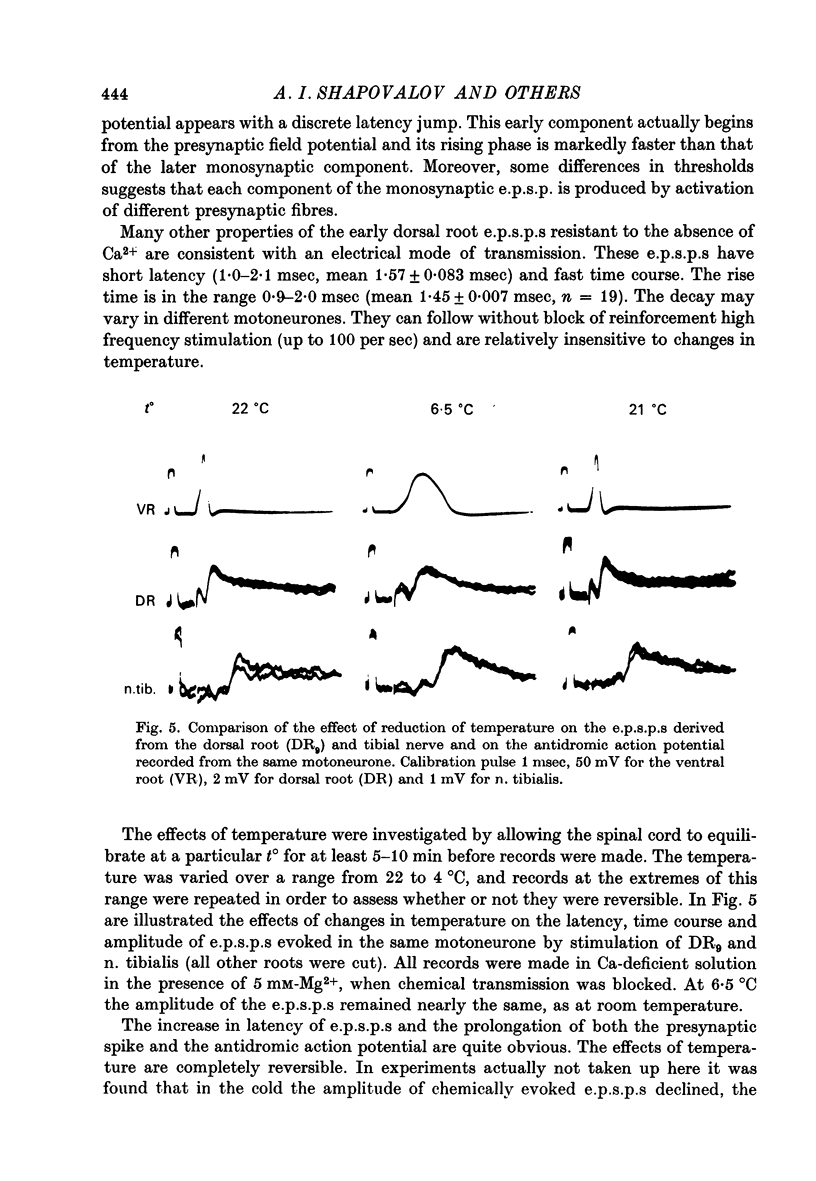






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKHART J. M., FADIGA E. Potential field initiated during monosynaptic activation of frog motoneurones. J Physiol. 1960 Mar;150:633–655. doi: 10.1113/jphysiol.1960.sp006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V. Electrical versus chemical neurotransmission. Res Publ Assoc Res Nerv Ment Dis. 1972;50:58–90. [PubMed] [Google Scholar]
- Cruce W. L. A supraspinal monosynaptic input to hindlimb motoneurons in lumbar spinal cord of the frog, Rana catesbiana. J Neurophysiol. 1974 Jul;37(4):691–704. doi: 10.1152/jn.1974.37.4.691. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A., Teb ecis A. K., Watkins J. C. Excitation of mammalian central neurones by acidic amino acids. Brain Res. 1972 Jun 22;41(2):283–301. doi: 10.1016/0006-8993(72)90503-3. [DOI] [PubMed] [Google Scholar]
- Dambach G. E., Erulkar S. D. The action of calcium at spinal neurones of the frog. J Physiol. 1973 Feb;228(3):799–817. doi: 10.1113/jphysiol.1973.sp010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. The effect of polarizing currents on unitary Ia excitatory post-synaptic potentials evoked in spinal motoneurones. J Physiol. 1976 Aug;259(3):705–723. doi: 10.1113/jphysiol.1976.sp011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FADIGA E., BROOKHART J. M. Monosynaptic activation of different portions of the motor neuron membrane. Am J Physiol. 1960 Apr;198:693–703. doi: 10.1152/ajplegacy.1960.198.4.693. [DOI] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D. A study of the interaction between motoneurones in the frog spinal cord. J Physiol. 1966 Feb;182(3):612–648. doi: 10.1113/jphysiol.1966.sp007841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. A STUDY OF SPONTANEOUS MINIATURE POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1963 Sep;168:389–422. doi: 10.1113/jphysiol.1963.sp007199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUBOTA K., BROOKHART J. M. RECURRENT FACILITATION OF FROG MOTONEURONS. J Neurophysiol. 1963 Nov;26:877–893. doi: 10.1152/jn.1963.26.6.877. [DOI] [PubMed] [Google Scholar]
- Kurchavyi G. G., Motorina M. V., Shapovalov A. I. Issledovanie raspredeleniia sinapticheskikh vkhodov na analogovoi modeli motoneironov. Neirofiziologiia. 1973 May-Jun;5(3):289–297. [PubMed] [Google Scholar]
- Nicholls J. G., Purves D. A comparison of chemical and electrical synaptic transmission between single sensory cells and a motoneurone in the central nervous system of the leech. J Physiol. 1972 Sep;225(3):637–656. doi: 10.1113/jphysiol.1972.sp009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Kurchavyi G. G. Effects of trans-membrane polarization and TEA injection on monosynaptic actions from motor cortex, red nucleus and group Ia afferents on lumbar motoneurons in the monkey. Brain Res. 1974 Dec 20;82(1):49–67. doi: 10.1016/0006-8993(74)90892-0. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I. Ionnye mekhanizmy vozbuzhdeniia postsinapticheskoi membrany neironov tsentral'noi nervnoi sistemy pozvonochnykh. Dokl Akad Nauk SSSR. 1975 Nov 11;225(2):477–479. [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I. Retikulo-spinal'nye i propriospinal'nye monosinapticheskie vliianiia na motoneirony liagushki. Neirofiziologiia. 1973 Mar-Apr;5(2):164–173. [PubMed] [Google Scholar]
- Sotelo C., Taxi J. Ultrastructural aspects of electrotonic junctions in the spinal cord of the frog. Brain Res. 1970 Jan 6;17(1):137–141. doi: 10.1016/0006-8993(70)90315-x. [DOI] [PubMed] [Google Scholar]
- Székely G. The morphology of motoneurons and dorsal root fibers in the frog's spinal cord. Brain Res. 1976 Feb 20;103(2):275–290. doi: 10.1016/0006-8993(76)90799-x. [DOI] [PubMed] [Google Scholar]
- Velumian A. A., Shapovalov A. I. Neodinakovaia chuvstvitel'nost' razlichnykh sinapticheskikh vkhodov motoneironov amfibii k nedostatku ionov kal'tsiia i ionam magniia. Dokl Akad Nauk SSSR. 1975 Nov 11;225(2):466–469. [PubMed] [Google Scholar]
- WATANABE A., GRUNDFEST H. Impulse propagation at the septal and commissural junctions of crayfish lateral giant axons. J Gen Physiol. 1961 Nov;45:267–308. doi: 10.1085/jgp.45.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weakly J. N. The action of cobalt ions on neuromuscular transmission in the frog. J Physiol. 1973 Nov;234(3):597–612. doi: 10.1113/jphysiol.1973.sp010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman R., Carlen P. L. Unusual behavior of the La EPSP in cat spinal motoneurons. Brain Res. 1976 Aug 13;112(2):395–401. doi: 10.1016/0006-8993(76)90294-8. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Puil E. A. Actions of glutamic acid on spinal neurones. Exp Brain Res. 1973 Mar 29;17(1):35–49. doi: 10.1007/BF00234562. [DOI] [PubMed] [Google Scholar]


