Abstract
A novel variant-specific surface protein (VSP) from Giardia was identified using the monoclonal antibody 9B10, raised against purified cyst walls. VSP9B10B is preferentially induced during encystation and expressed simultaneously with other VSPs on the surface of encysting trophozoites. These results support the hypothesis that encystation and antigenic variation are processes that are mechanistically related.
Giardia lamblia is a flagellated protozoan that resides in the upper small intestine of humans and other mammals. Susceptible hosts become infected by ingestion of the environmentally resistant cysts that, after excystation in the duodenum, release the motile trophozoites that colonize and multiply, attached to the intestinal mucosa (1). Giardia has developed two distinctive mechanisms of defense against the hostile environmental conditions that the parasite confronts during its life cycle, the processes known as antigenic variation (5, 12) and encystation (5, 6). These two adaptive responses have been recognized in Giardia for a long time, but the molecular basis underlying these processes is poorly understood.
Giardia differentiation into cysts is essential for the parasite to survive outside the host's intestine since trophozoites are very susceptible to changes in temperature, humidity, and ionic strength and to chemical disinfectants (1). This process initiates when trophozoites reach the lower parts of the small intestine and sense low cholesterol concentrations in the environment (7) and culminates with the formation of a carbohydrate-rich extracellular cyst wall that protects the parasite under relatively harsh conditions (4, 5, 6).
Antigenic variation, on the other hand, is a mechanism by which the trophozoites changes their surface coat to survive inside the host's intestine and to cause chronic and recurrent infections (1, 12). At a given point in time, it was assumed that an individual trophozoite is covered by only one member of a family of antigenically diverse proteins called variant-specific surface proteins (VSPs) (12, 16). Spontaneously or in response to the host's immune system, one VSP may be replaced by other antigenically distinct VSP on the surface of the trophozoites by as-yet-unknown mechanisms (12).
Meng et al. (10) have proposed a correlation between encystation and antigenic variation in Giardia based in the observation that trophozoites covered by a specific VSP have lost that surface protein after encystation in vitro. Subsequently, Svärd et al. (17) reported that during differentiation into cysts, the predominant VSP diminishes and is internalized into lysosome-like peripheral vacuoles, at the time that transcripts encoding different VSPs begin to appear.
Recently, Nash et al. (15) showed that that a monoclonal antibody (MAb) called 9B10 reacts to the surface and flagella of some trophozoites and cloned the gene that encodes that VSP (VSP9B10; GenBank accession no. AAG16629 [hereafter called VSP9B10A]). In that work, Nash et al. reported for the first time the expression of two distinct VSPs in a single trophozoite and suggested that, similar to other protozoa that undergo antigenic variation, this observation was due to the fact that one surface antigen is replaced by another VSP over a relatively long period of time (15).
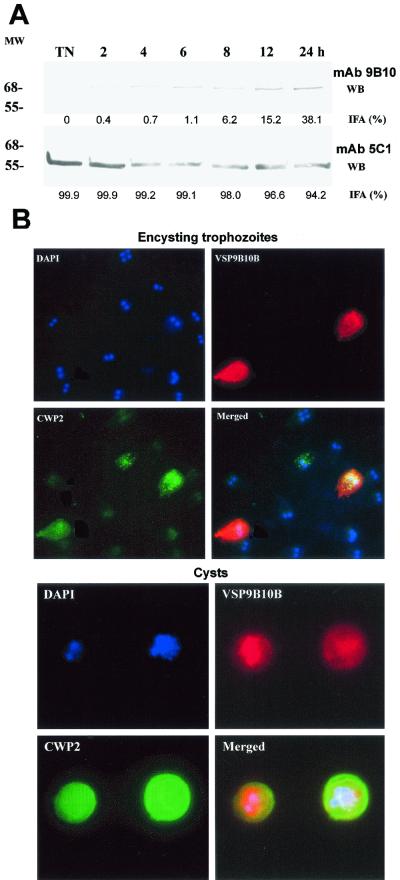
Since we found that several Giardia isolates obtained from feces of infected individuals from Argentina expressed VSP9B10 on their surface (see bellow) and the fact that the MAb 9B10 was produced by immunizing mice with purified cyst walls from in vitro-derived cysts of the clone WB/1267 (8), we hypothesized that this VSP could be preferentially expressed during trophozoite differentiation into cysts. To test this possibility, we first used the MAb 9B10 in immunofluorescence assays (IFA) and Western blotting of trophozoites induced to encyst for different periods of time. Since Giardia trophozoites in culture may express different VSPs, in this work we have always used recently cloned parasites (lest than 5 days in culture after cloning). When not specified, methods and techniques were as previously reported (8, 9, 15, 19). Our results show that in vegetative trophozoites of the clone WB/1267, the MAb 9B10 does not recognize any antigen either by IFA or Western blotting. In encysting trophozoites, however, MAb 9B10 detects a protein of ∼68 kDa, slightly different from the molecular mass of the protein predicted by the VSP9B10A gene (76 kDa), the expression of which increases more than 30-fold at 24 h of encystation compared to the expression of VSP1267 recognized by the MAb 5C1 (14) (Fig. 1A). Conversely, no such increase in expression was observed on trophozoites maintained for 24 h in growth medium (not shown). In addition, the percentage of trophozoites expressing VSP9B10 on their surface detected by IFA also augmented from 0% in nonencysting trophozoites to reach a maximum of 38% at 24 h (Fig. 1A). Interestingly, the percentage of cells expressing VSP1267 only changed from 99 to 91% during the same period, indicating that during this process at least these two VSPs were expressed at the same time in a single trophozoite (not shown). Figure 1B shows an immunofluorescence assay with encysting trophozoites and in vitro-derived cysts using fluorescein-labeled MAb 7D2 (specific for cyst wall protein 2 [CWP2] [8]), rhodamine-labeled MAb 9B10 (specific for VSP9B10), and counterstaining with the DNA-labeling fluorogenic dye 4′,6′-diamidino-2-phenylindole (DAPI). Results indicated that VSP9B10 is preferentially expressed in those trophozoites undergoing encystation, as demonstrated by the presence of encystation-specific vesicles containing CWP2 (top panel of Fig. 1B). Cysts generated in vitro from clone WB1267 containing one (left-hand panels, two nuclei labeled with DAPI) or two (right-hand panels, four nuclei) trophozoites expressing VSP9B10 are shown at the bottom of Fig. 1B. Similar observations were obtained when clones belonging to Giardia assemblage A were used (WB/C6 and WB/A6), initially described as Giardia group 1 isolates (13, 18). In contrast, no expression of this novel VSP was observed in the clone GS/H7, which belongs to group 3 (assemblage B) (13, 18), indicating that switching to VSP9B10 is not universal for all Giardia isolates.
FIG. 1.
(A) Western blots of Giardia WB1267 trophozoites undergoing encystation from 0 (NT) to 24 h using MAb 9B10 (specific for VSP9B10) and 5C1 (specific for VSP1267). Figures under each lane represent the percentage of reactive cells as determined by immunofluorescence assays (values are the mean of three independent experiments performed in duplicate). Molecular weight (MW) is shown at left (in thousands). (B) Immunofluorescence assays of encysting trophozoites (top) and in vitro-derived cysts (bottom).
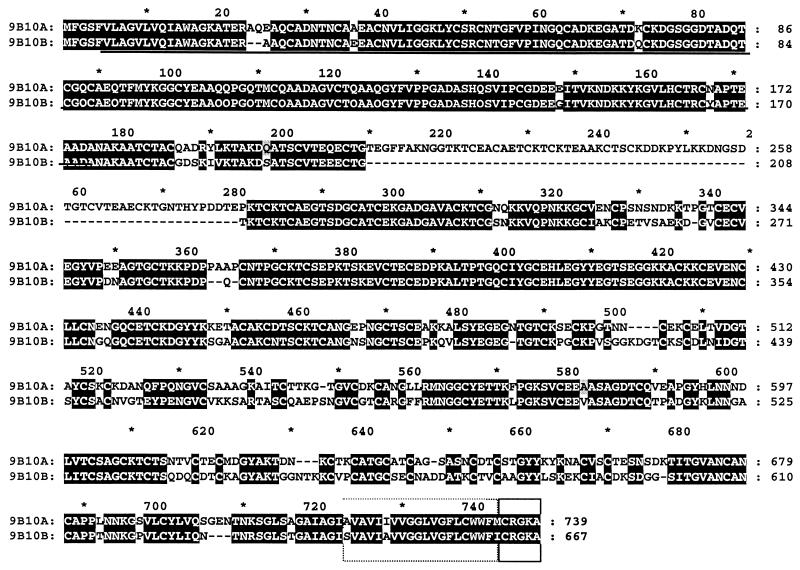
These results prompted us to verify whether the MAb 9B10 recognizes different VSPs. By immunoscreening with the MAb 9B10 of a cDNA library constructed with mRNA extracted from trophozoites of the clone WB/1267 induced to encyst from different periods of time (8), we were able to identify three different plaques, which were purified, and their inserts were subcloned and sequenced as reported (8, 9, 19). The sequences of these clones corresponded to slightly different fragments of a sequence that when subjected to BLAST analysis (3) showed high homology to the amino terminus of several known Giardia VSPs. Then, we used the inserts present in those clones to screen a genomic DNA library generated from genomic DNA also belonging to the clone WB/1267 (8). Cloning and sequencing of seven isolated clones allowed the assembly of the complete sequence of two different VSP genes (the previously reported VSP9B10A and a different VSP that we called VSP9B10B; GenBank accession no. AF293416). VSP9B10 genes codify acidic, cysteine-rich proteins of 76.763 (VSP9B10A) and 68.451 (VSP9B10B) daltons. The presence of these two 9B10 genes in the Giardia genome was confirmed by Southern blotting assays (not shown). The molecular mass predicted by 9B10B is in agreement with the one recognized by the MAb 9B10 in Western blots (Fig. 1). Comparative analysis of the amino acid sequences predicted by both VSP9B10 genes show that they may derive from a common ancestor (1, 2, 12): they contain highly homologous N-terminal and C-terminal domains and a more divergent central region where VSP9B10A contains an insertion of ∼70 amino acids (Fig. 2). Figure 2 also shows the common region recognized by MAb 9B10 (underlined) as deduced by analysis of the plaques isolated by immunoscreening of a cDNA library using that antibody, the transmembrane region (broken box), and the conserved cytosolic tail (closed box). Although it was previously shown that a MAb might recognize diverse VSPs due to the presence of different number of repeating epitopes in VSPA6 (11), it seems not to be the case for MAb 9B10. Northern blotting of encysting trophozoites from different Giardia clones probed with 32P-labeled both full-length VSP9B10B gene and the VSP9B10A insertion (amplified with primers A-S, 5′-CAAGCCGTACCTGAAGAAGG-3′, and A-AS, 5′-TCGTCGGGGTAGTGGGTG-3′) only showed the presence of the VSP9B10B transcript during encystation (Fig. 3), which further supports the fact that VSP9B10 is only present in Giardia assemblage A isolates.
FIG. 2.
Alignment of the deduced amino acid sequences of VSP9B10A and VSP9B10B.
FIG. 3.
Northern blot on RNA extracted from encysting trophozoites of clones WB/1267, WB/C6, WB/9B10, and GS/H7 using probes specific for VSP9B10B (left) and VSP9B10A (right). Molecular size markers are indicated (in nucleotides).
Additionally, to investigate whether VSP9B10B is expressed by parasites isolated from feces of infected humans, cysts were harvested and excysted in vitro (10, 17). Then, IFA using MAb 9B10 and several other MAbs specific to different VSPs was performed on trophozoites. Our results demonstrated that VSP9B10 is present in trophozoites derived from naturally infected persons, and clones 100% expressing VSP9B10B were isolated and maintained in culture (not shown). Until this time, we were unable to find a link between the clinical manifestations of the infection in those individuals and this particular VSP.
In summary, we have showed here that during Giardia trophozoite differentiation into cyst, a novel VSP is preferentially expressed on the surface of parasites belonging to Giardia assemblage A (group 1). VSP9B10B colocalizes with other VSPs on the plasma membrane of encysting trophozoites at least during differentiation and in in vitro- and in vivo-derived cysts. These results strongly support previous observations that antigenic variation and differentiation are processes that are mechanistically related (5, 10). In addition, we provide new evidence that Giardia assemblages A and B are markedly divergent and that VSP9B10 can be used to discriminate between Giardia isolates belonging to different Giardia groupings.
Acknowledgments
This work was supported by the Agencia Nacional para la Promocion de la Ciencia y la Tecnologia (ANPCYT), Fundacion Antorchas, Universidad Nacional de Cordoba, Consejo Nacional de Investigaciones Cientificas y Tecnicas (CONICET), and the Howard Hughes Medical Institute.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Adam, R. D. 2001. Biology of Giardia lamblia. Clin. Microbiol. Rev. 35:1332-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam, R. D. 2000. The Giardia lamblia genome. Int. J. Parasitol. 30:475-484. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erlandsen, S. L., P. T. Macechko, H. Van Keulen, and E. Jarroll. 1996. Formation of Giardia cyst wall: studies on extracellular assembly using immunogold labeling and high resolution field emission SEM. J. Eukaryot. Microbiol. 43:416-429. [DOI] [PubMed] [Google Scholar]
- 5.Gillin, F. D., D. S. Reiner, and J. M. McCaffery. 1996. Cell biology of the primitive eukaryote Giardia lamblia. Annu. Rev. Microbiol. 50:679-705. [DOI] [PubMed] [Google Scholar]
- 6.Luján, H. D., M. R. Mowatt, and T. E. Nash. 1997. The mechanisms of Giardia lamblia differentiation into cysts. Microbiol. Mol. Biol. Rev. 61:294-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luján, H. D., M. R. Mowatt, L. G. Byrd, and T. E. Nash. 1996. Cholesterol starvation induces differentiation of the intestinal parasite Giardia lamblia. Proc. Natl. Acad. Sci. USA 93:7628-7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luján, H. D., M. R. Mowatt, J. T. Conrad, B. Bowers, and T. E. Nash. 1995. Identification of a novel Giardia lamblia cyst wall protein with leucine-rich repeats. J. Biol. Chem. 270:29307-29313. [DOI] [PubMed] [Google Scholar]
- 9.Luján, H. D., M. R. Mowatt, J. T. Conrad, and T. E. Nash. 1996. Increased expression of the molecular chaperone BiP/GRP78 during the differentiation of a primitive eukaryote. Biol. Cell 86:11-18. [DOI] [PubMed] [Google Scholar]
- 10.Meng, T. C., M. J. Hetsko, and F. D. Gillin. 1993. Antigenic switching of TSA 417, a trophozoite variable surface protein, following completion of the life cycle of Giardia lamblia. Infect. Immun. 61:5394-5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mowatt, M. R., B. Y. Nguyen, J. T. Conrad, R. D. Adam, and T. E. Nash. 1994. Size heterogeneity among antigenically related Giardia lamblia variant-specific surface proteins is due to differences in tandem repeat copy number. Infect. Immun. 62:1213-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nash, T. E. 1997. Antigenic variation in Giardia lamblia and the host's immune response. Phil. Trans. R. Soc. Lond. B 352:1369-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nash, T. E. 1992. Surface antigen variability and variation in Giardia lamblia. Parasitol. Today 8:229-234. [DOI] [PubMed] [Google Scholar]
- 14.Nash, T. E., J. T. Conrad, and J. W. Merritt. 1990. Variant specific epitopes of Giardia lamblia. Mol. Biochem. Parasitol. 42:124-132. [DOI] [PubMed] [Google Scholar]
- 15.Nash, T. E., H. D. Luján, M. R. Mowatt, and J. T. Conrad. 2001. Variant-specific surface protein switching in Giardia lamblia. Infect. Immun. 69:1922-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pimenta, P. F. P., P. P. da Silva, and T. E. Nash. 1991. Variant surface antigens of Giardia lamblia are associated with the presence of a thick cell coat: thin section and label fracture immunocytochemistry survey. Infect. Immun. 59:3889-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svärd, S. G., T. C. Meng, M. L. Hetsko, J. M. McCaffery, and F. D. Gillin. 1998. Differentiation-associated surface antigen variation in the ancient eukaryote Giardia lamblia. Mol. Microbiol. 30:979-989. [DOI] [PubMed] [Google Scholar]
- 18.Thompson, R. C. A., R. M. Hopkins, and W. L. Homan. 2000. Nomenclature and genetic grouping of Giardia infecting animals. Parasitol. Today 16:210-213. [DOI] [PubMed] [Google Scholar]
- 19.Touz, M. C., M. J. Nores, I. Slavin, C. Carmona, J. T. Conrad, M. R. Mowatt, Nash, T. E., C. E. Coronel, and H. D. Luján. 2002. The activity of a developmentally regulated cysteine proteinase is required for cyst wall formation in the primitive eukaryote Giardia lamblia. J. Biol. Chem. 277:8474-8481. [DOI] [PubMed]