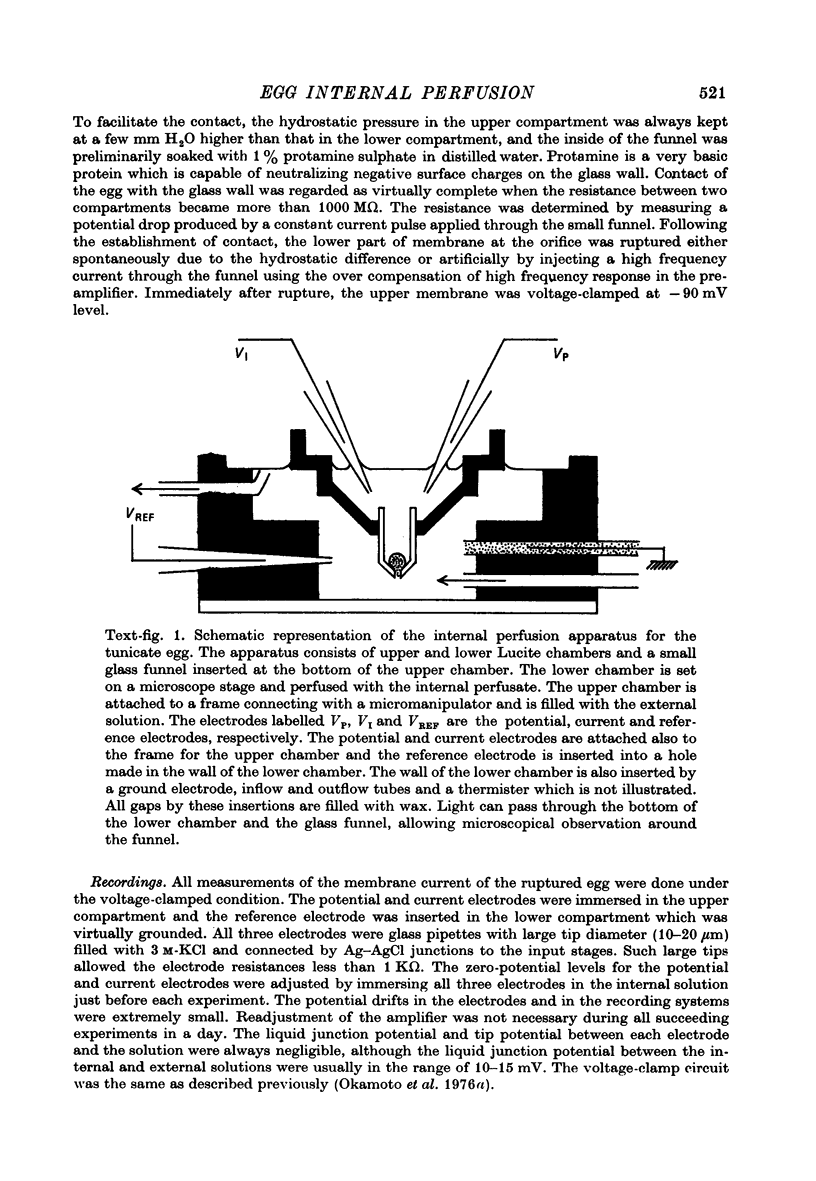
Abstract
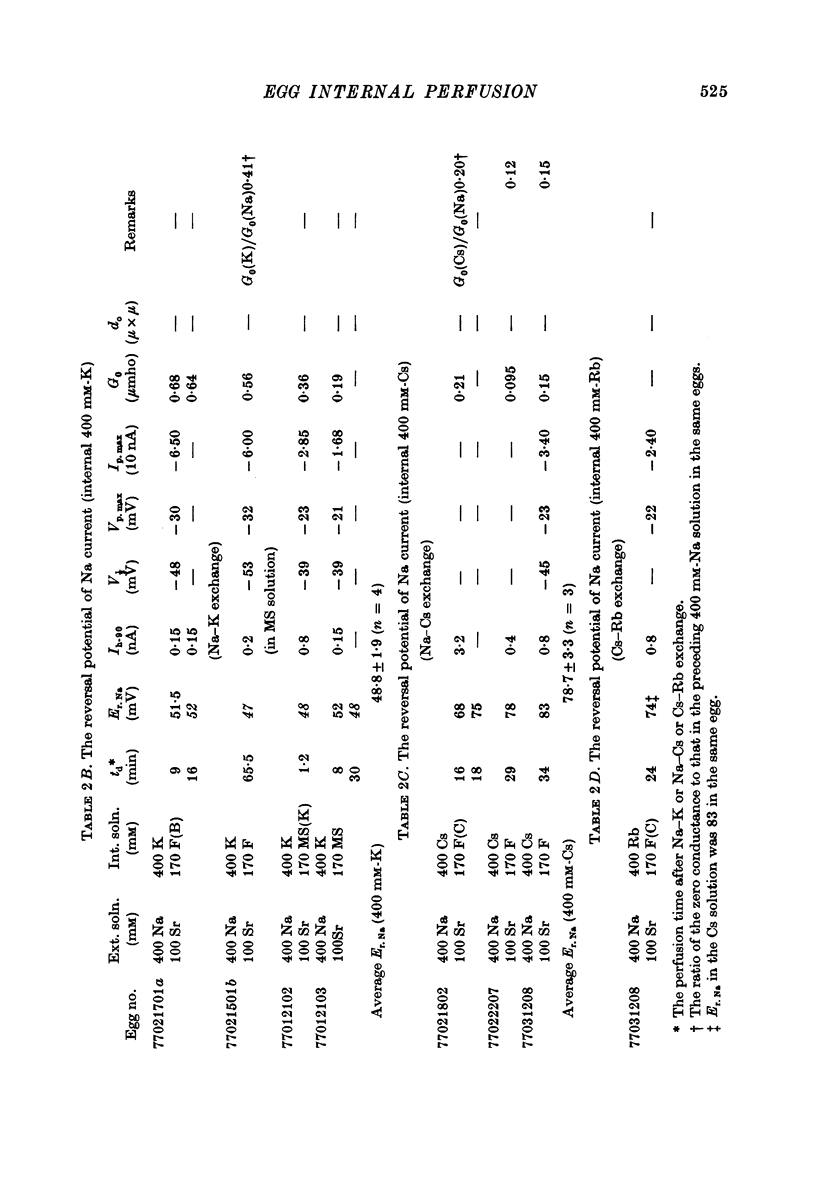
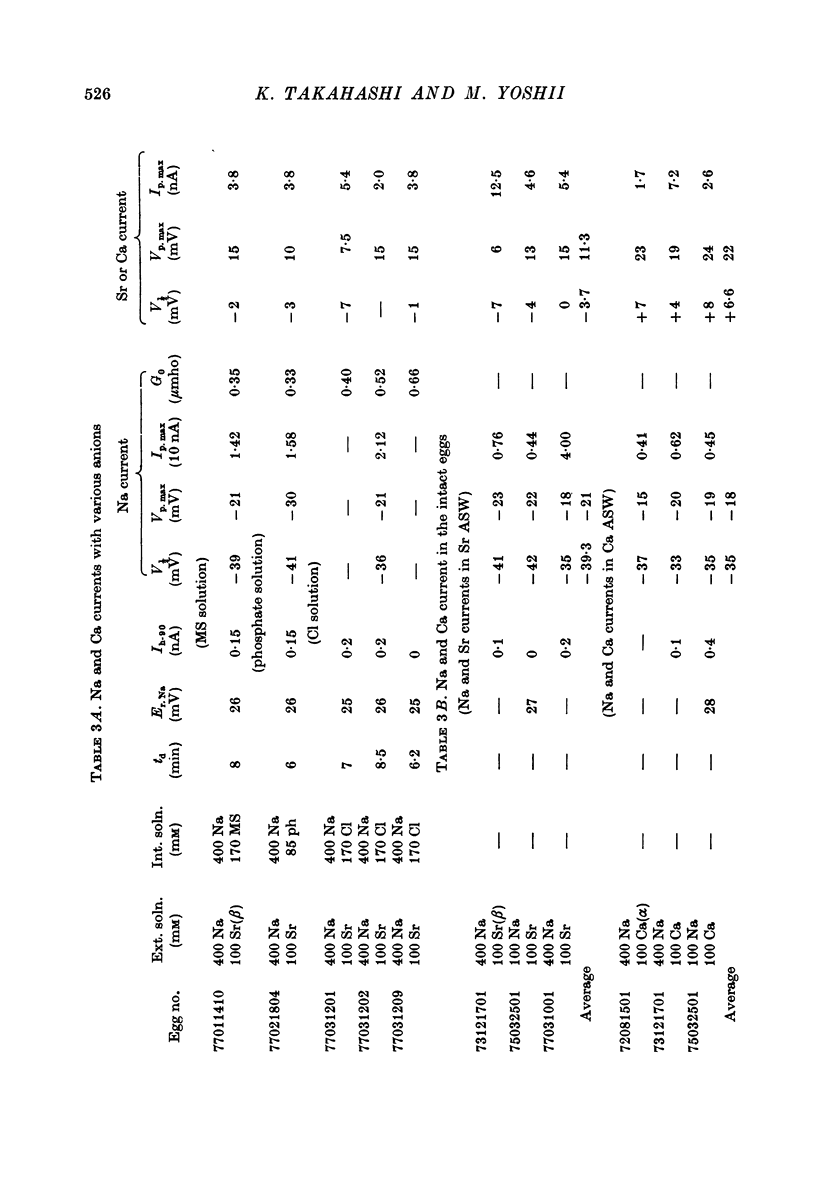
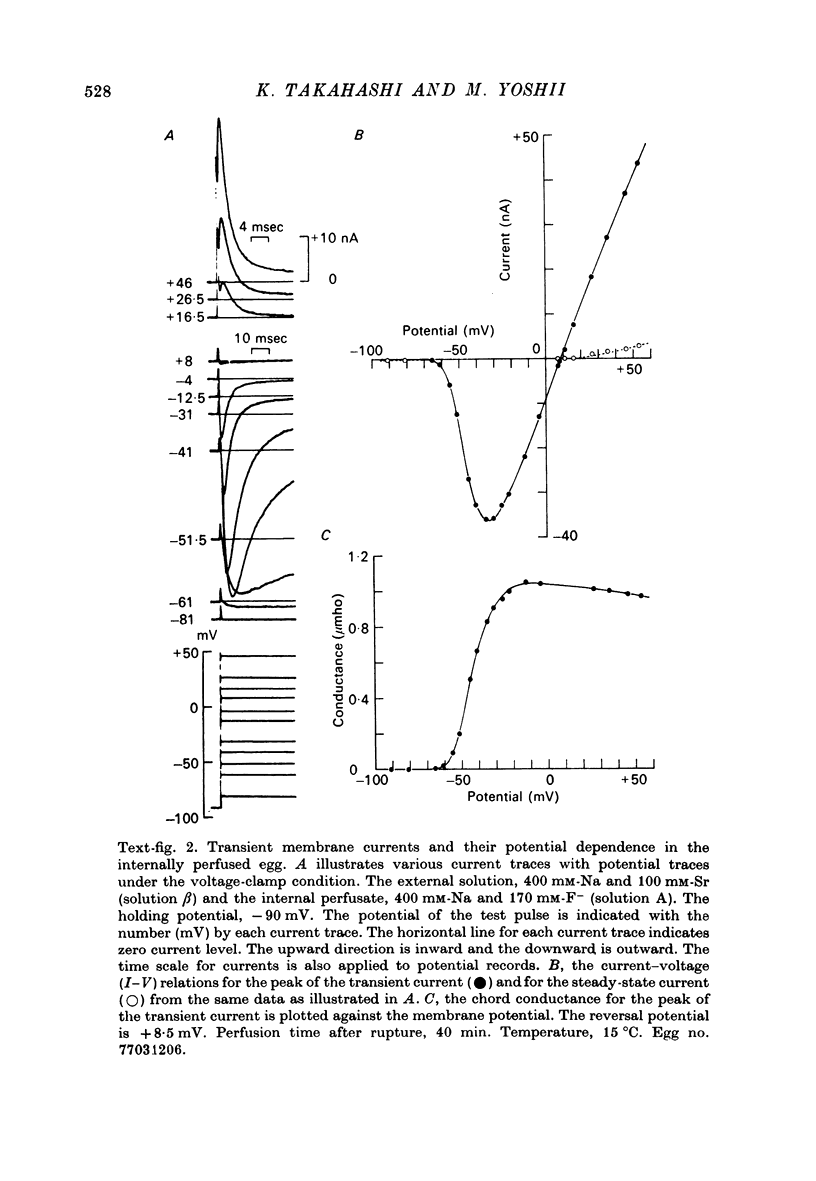
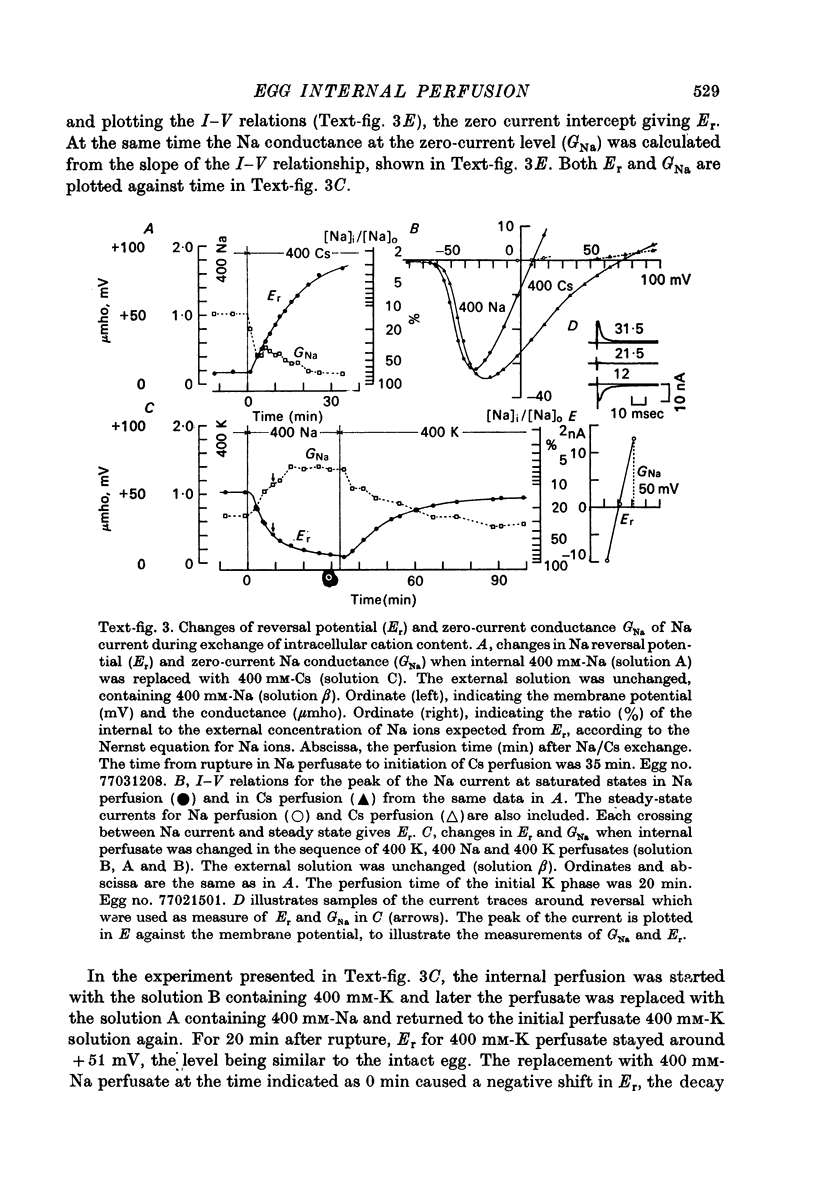
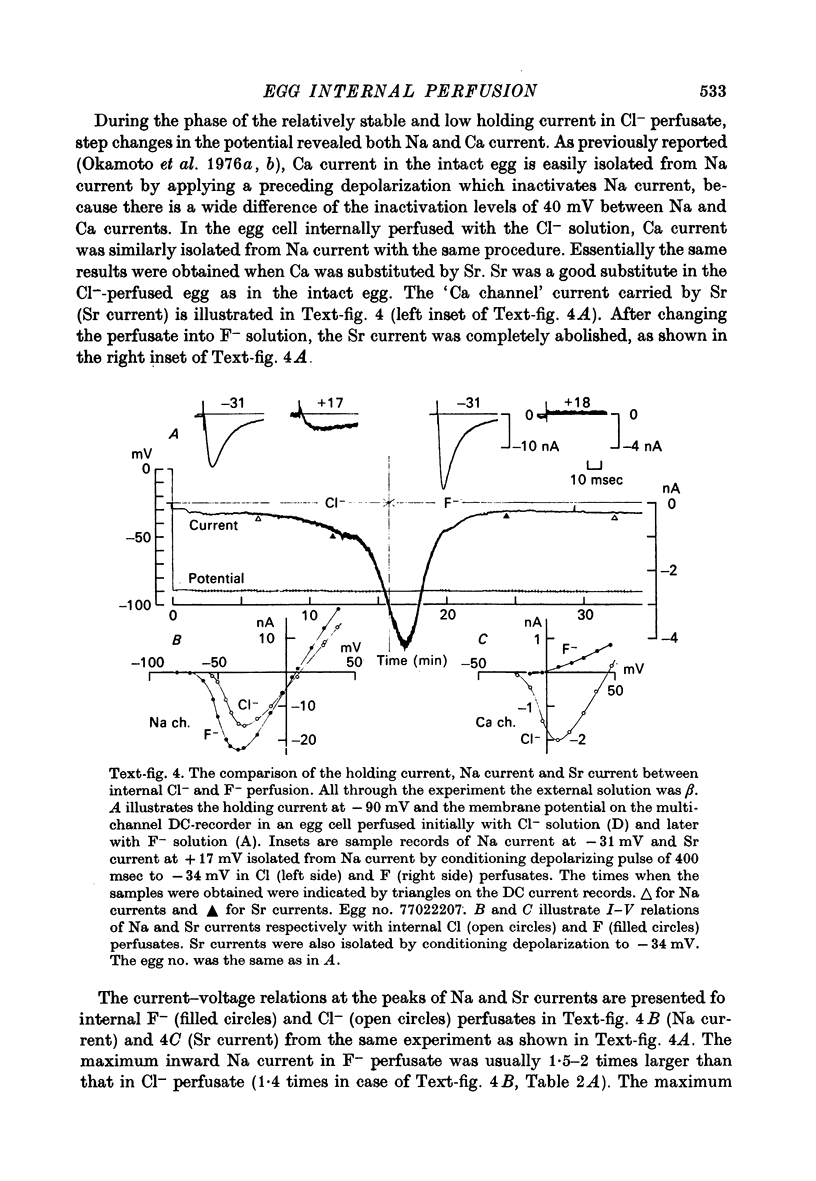
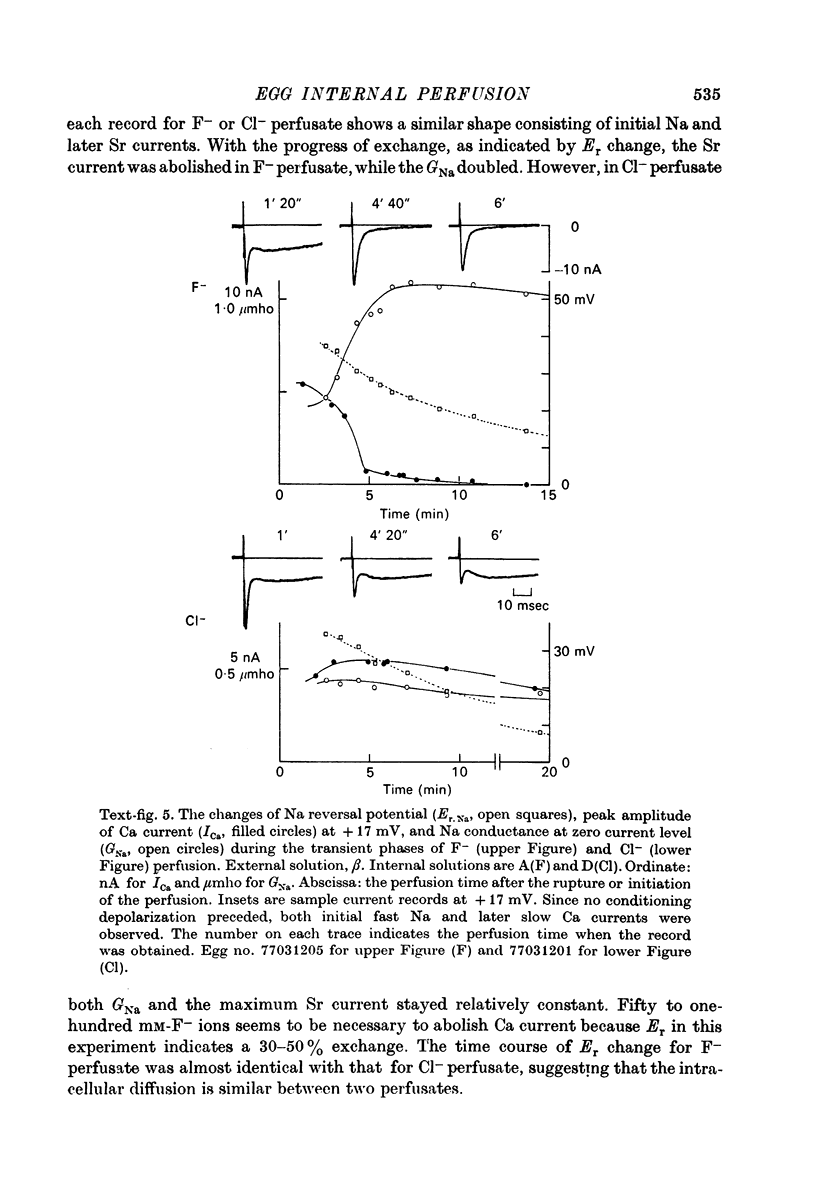
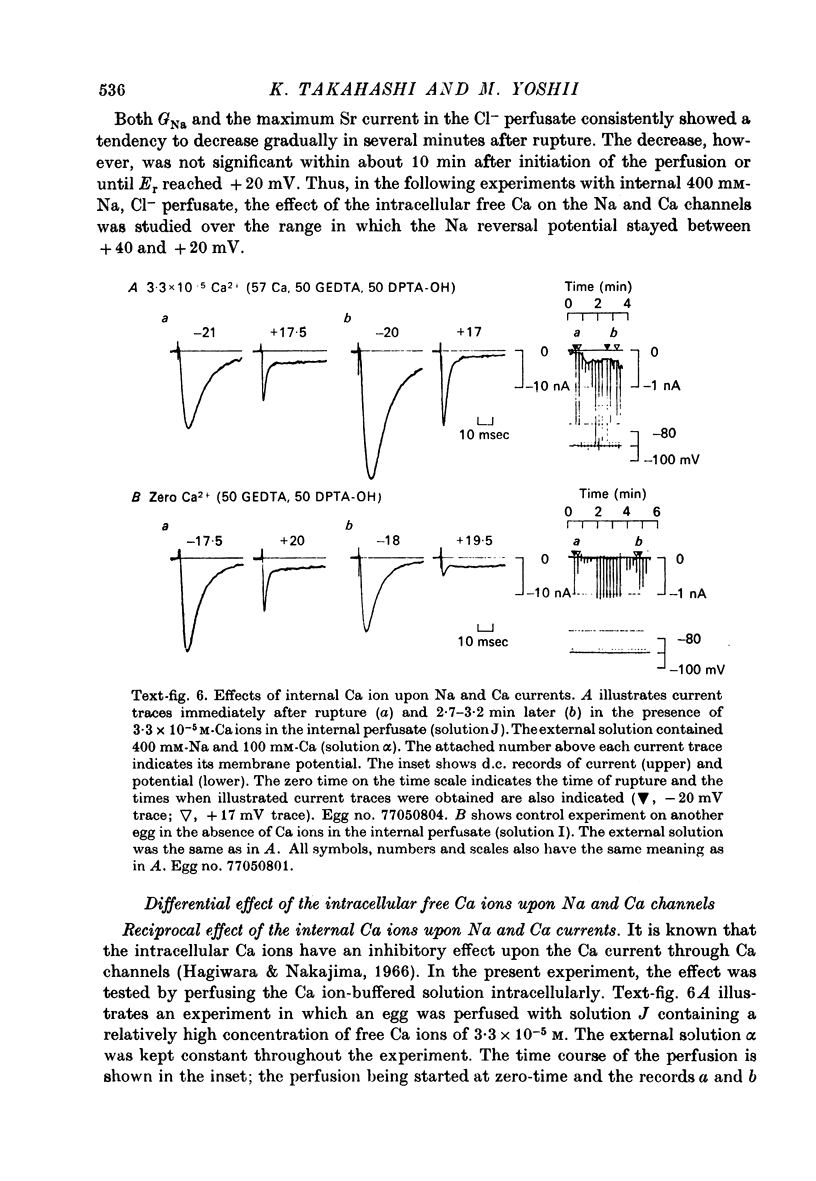
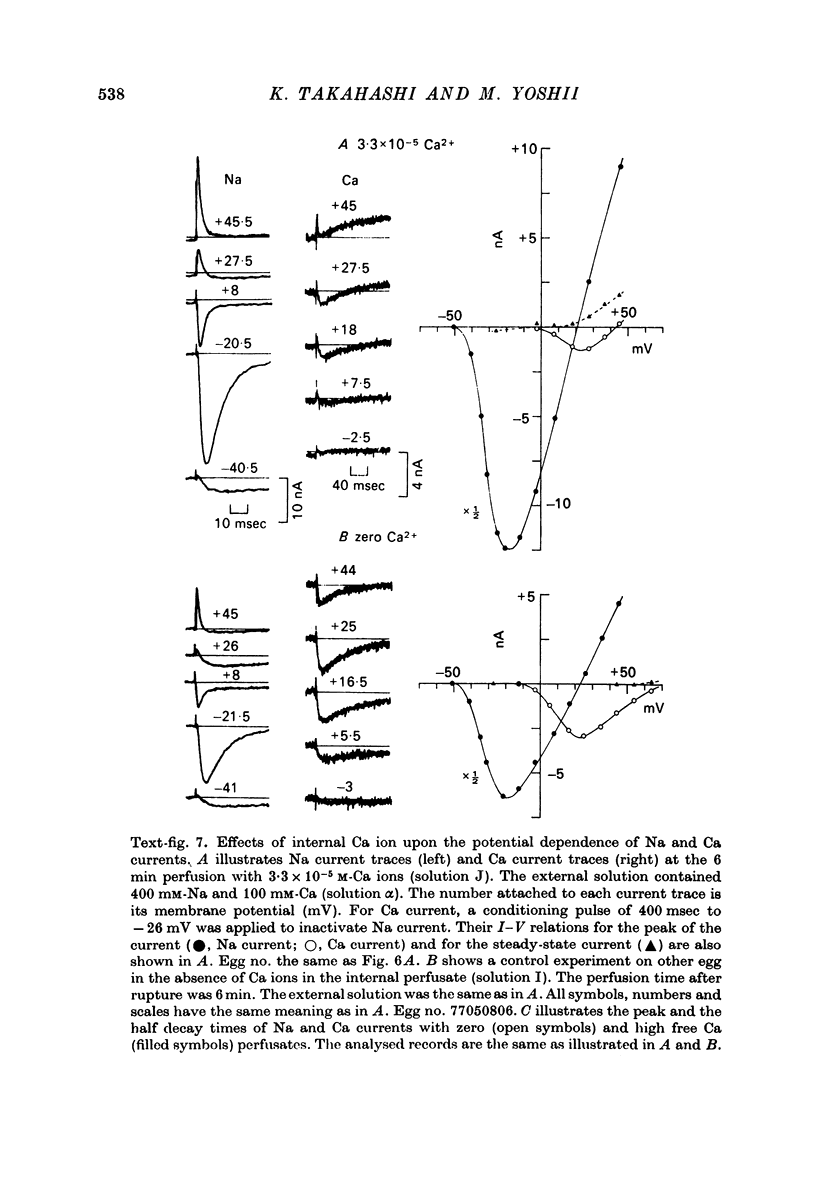
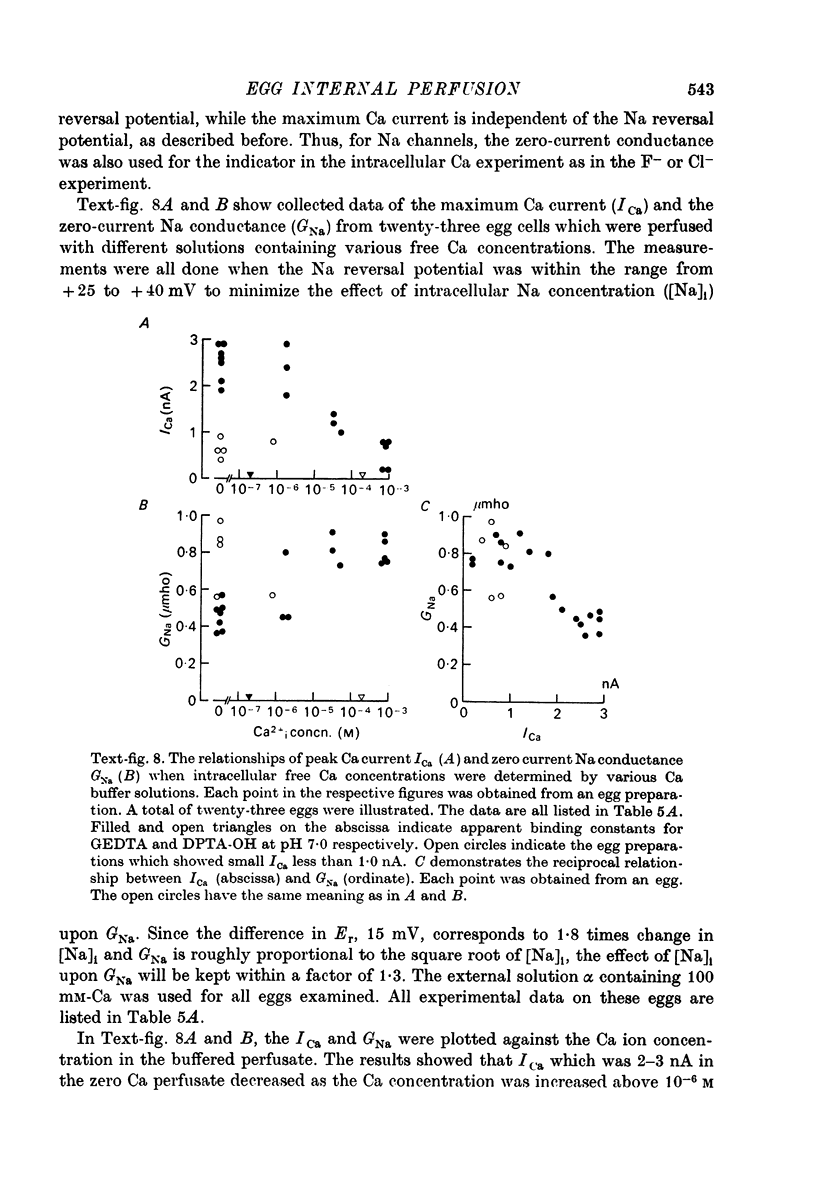
1. The unfertilized egg of the tunicate, Halocynthia roretzi, was intracellularly perfused with various solutions. 2. The perfusion apparatus consisted of lower and upper compartments which were connected by a small glass funnel. A denuded egg cell without chorion was dropped into the funnel and brought into close contact with the glass wall of the funnel. The membrane of the egg faced to the lower compartment was ruptured by a slight difference of hydrostatic pressure and the inside of the egg was perfused with the internal solution flowing through the lower compartment. The current across the upper membrane was analysed by voltage-clamp technique. 3. The egg cell in contact with 400 mM-Na external solution and perfused intracellularly with 400 mM-Na for 30 min showed a relatively low Na reversal potential, +6 mV, in comparison with +60 mV in the intact egg in standard artificial sea water. The exchange efficiency was monitored by observing the shift of Na reversal potential during perfusion with high Na internal perfusate. 4. The internal perfusate containing F- ions stabilized the egg membrane and kept the excitability for 1--2 hr during the intracellular perfusion. With the internal F- perfusate the intracellular cationic content was changed to 400 mM-Na, K, Rb or Cs (external solution of 400 mM-Na) and permeability ratios of the egg Na channel were estimated as PNa:PK:PRb:PCs=1.0:0.14:0.05:0.04. The internal F- perfusate abolished Ca current which was consistently observed in the intact egg, while the internal Cl- perfusate kept both Na and Ca current as in the intact egg. However with the internal Cl- perfusate the egg cell could not be kept in good condition more than 20-30 min. 6. The effects of intracellular free Ca ions upon the egg Na and Ca channels were analysed by using Ca ion-buffered internal Cl- and high Na perfusate. The results showed that internal Ca ions above 10(-6) reduced the Ca current and enhanced the Na current at the same time. In the range between 10(-5) and 10(-4) M the Ca current became half of the control obtained with zero free Ca perfusate while the Na conductance at the zero current level doubled. The internal Ca ions above one mM seemed to abolish the Ca current and to reduce the Na current as well. The reciprocal effect of intracellular Ca ions upon the egg Na and Ca channels was demonstrated in the concentration range from 10(-6) to 10(-3) M.
Full text
PDF


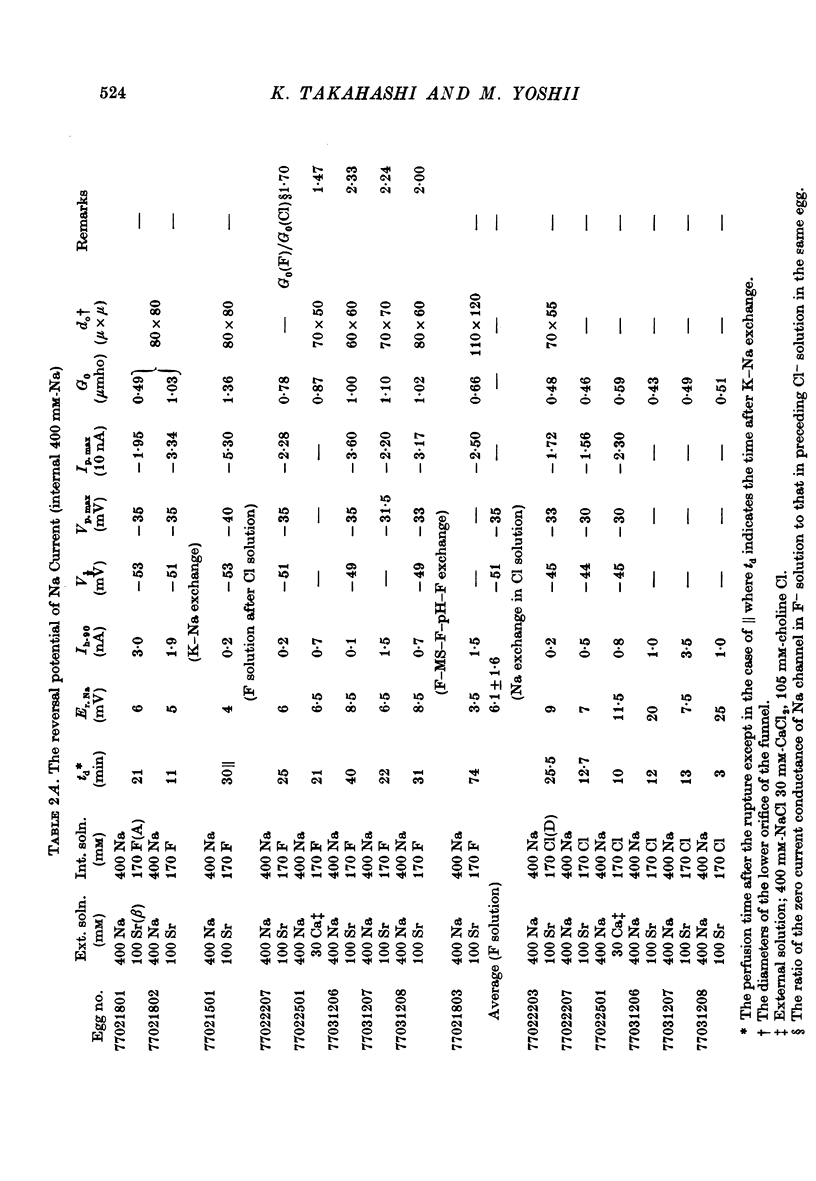






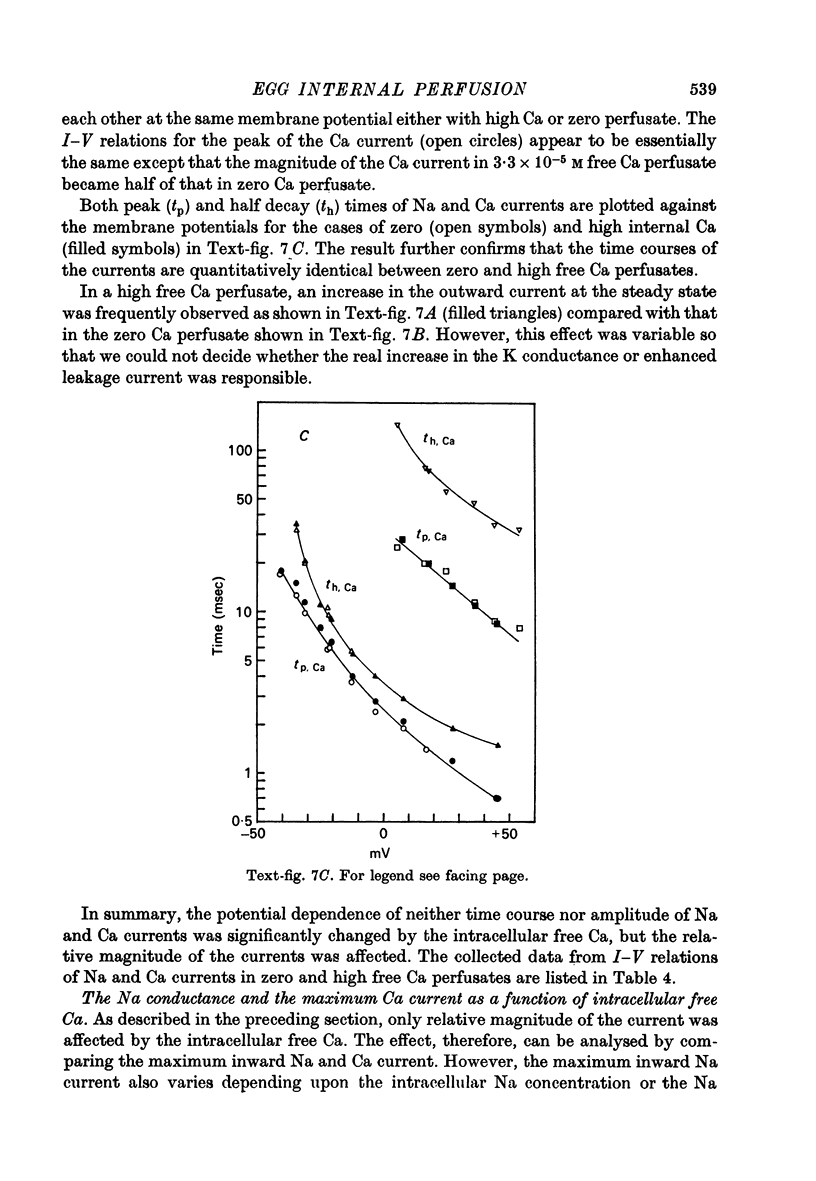
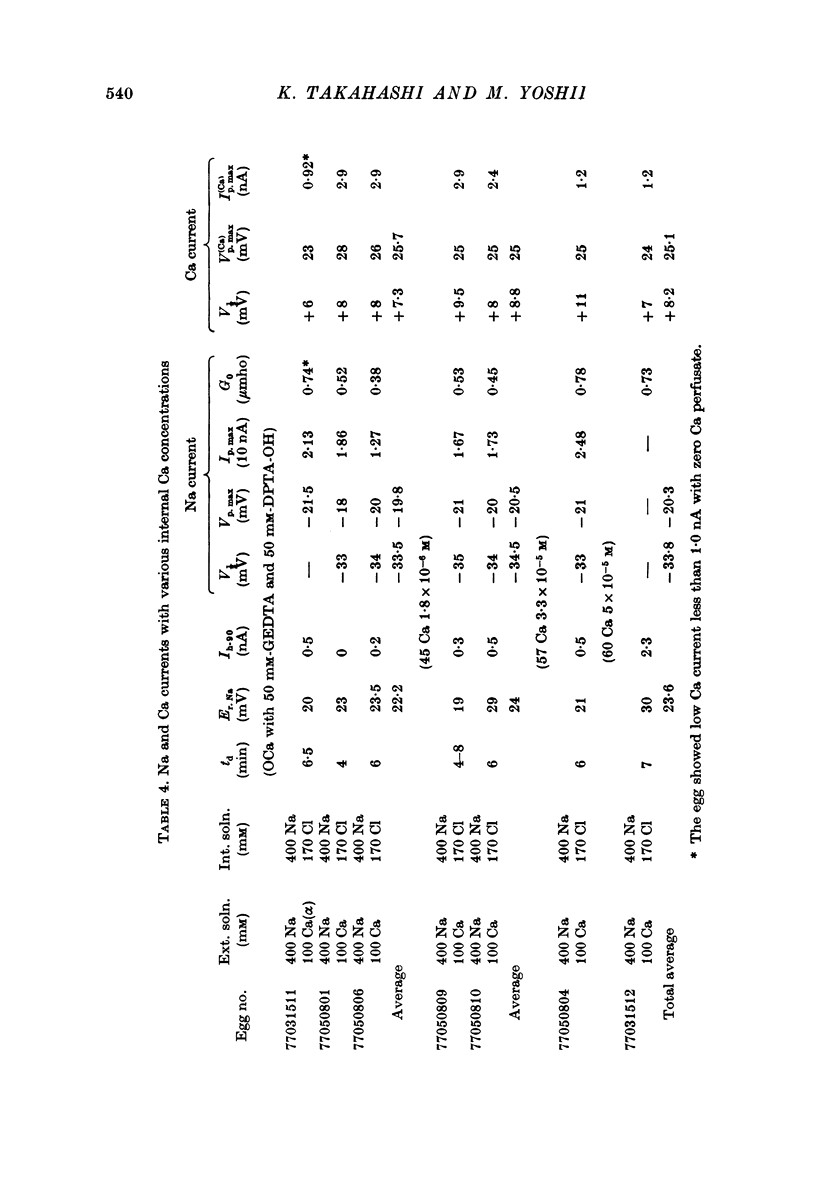
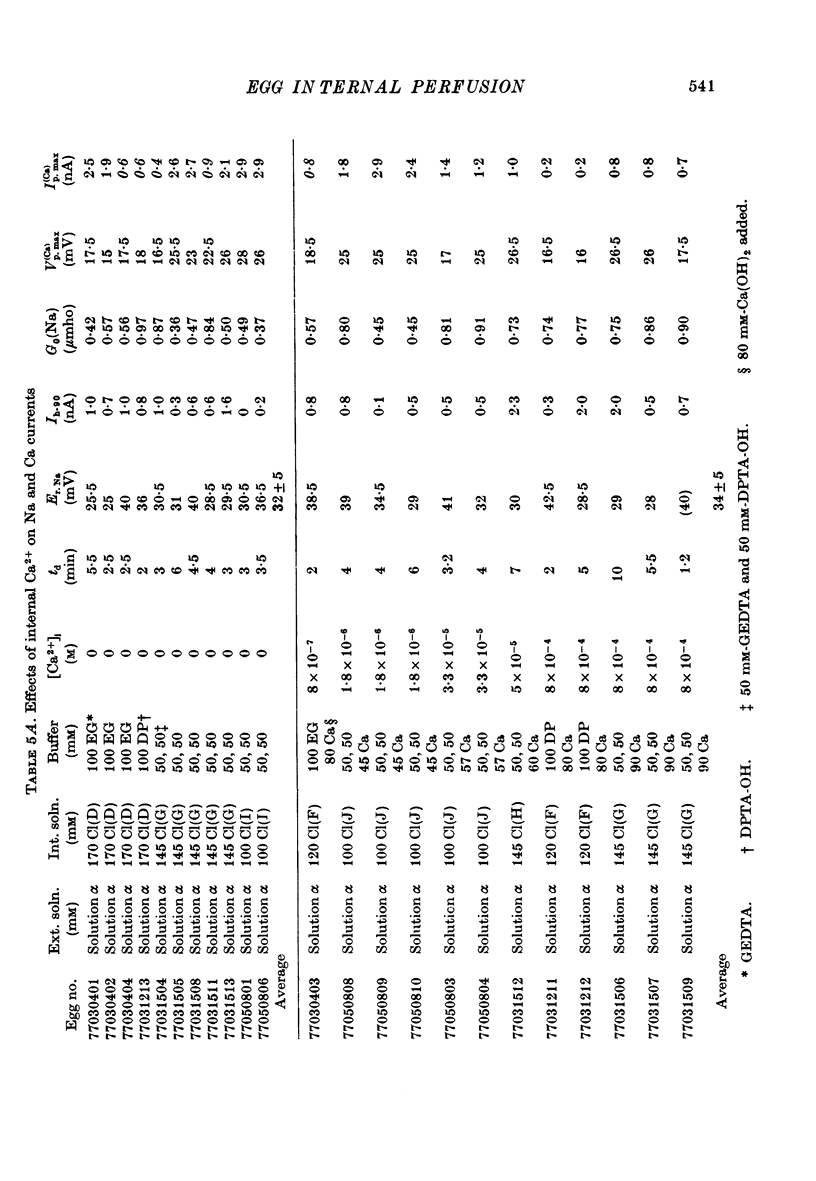
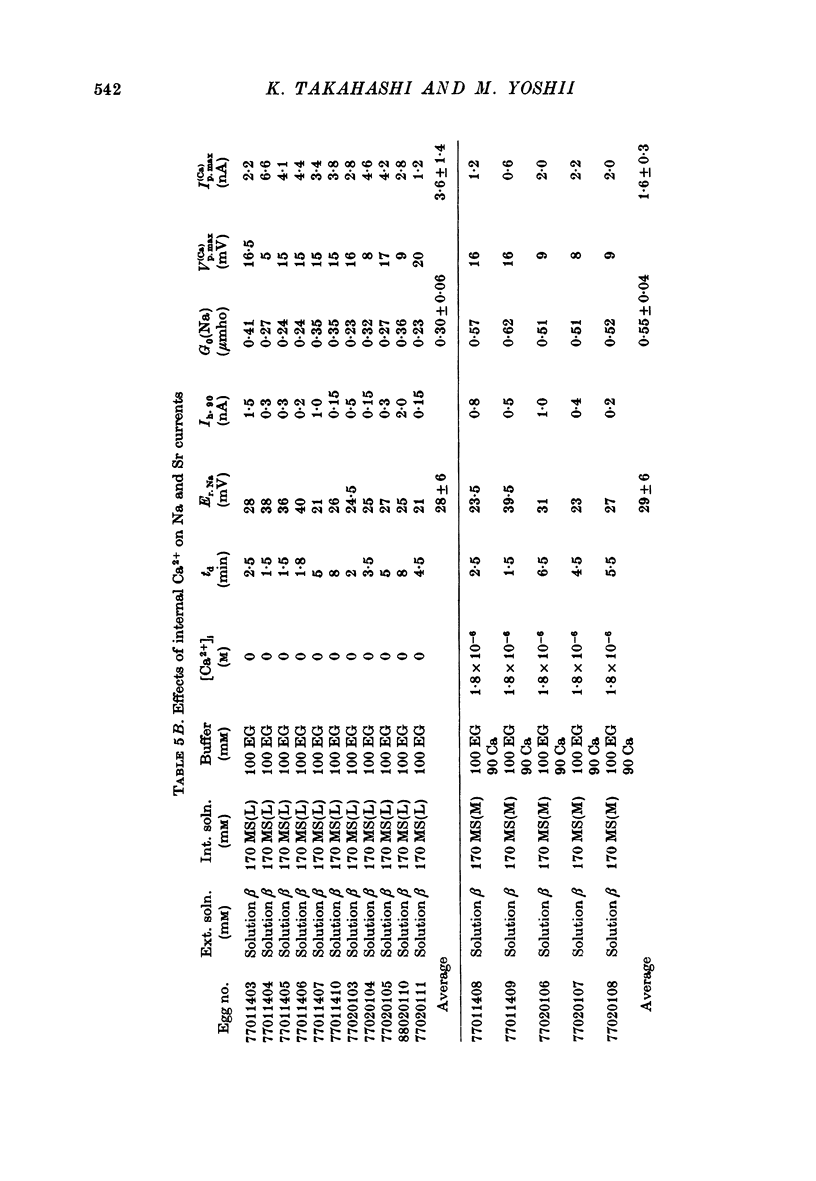






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T., Lynch C. Effects of internal divalent cations on voltage-clamped squid axons. J Gen Physiol. 1974 Jun;63(6):675–689. doi: 10.1085/jgp.63.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. T. Ionic selectivity of the sodium channel of frog skeletal muscle. J Gen Physiol. 1976 Mar;67(3):295–307. doi: 10.1085/jgp.67.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P. Axoplasmic free magnesium levels and magnesium extrusion from squid giant axons. J Gen Physiol. 1976 Aug;68(2):159–178. doi: 10.1085/jgp.68.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Turnover of acetylcholine receptors in skeletal muscle. Cold Spring Harb Symp Quant Biol. 1976;40:237–251. doi: 10.1101/sqb.1976.040.01.025. [DOI] [PubMed] [Google Scholar]
- Ebert G. A., Goldman L. The permeability of the sodium channel in Myxicola to the alkali cations. J Gen Physiol. 1976 Sep;68(3):327–340. doi: 10.1085/jgp.68.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Effects of the intracellular Ca ion concentration upon the excitability of the muscle fiber membrane of a barnacle. J Gen Physiol. 1966 Mar;49(4):807–818. doi: 10.1085/jgp.49.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Kano M., Yamamoto M. Development of spike potentials in skeletal muscle cells differentiated in vitro from chick embryo. J Cell Physiol. 1977 Mar;90(3):439–444. doi: 10.1002/jcp.1040900307. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y. Sodium and calcium components of the action potential in a developing skeletal muscle cell line. J Physiol. 1975 Jan;244(1):145–159. doi: 10.1113/jphysiol.1975.sp010788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A. Effects of calcium and calcium-chelating agents on the inward and outward current in the membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):569–580. doi: 10.1113/jphysiol.1977.sp011969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Asymmetrical displacement currents in nerve cell membrane and effect of internal fluoride. Nature. 1977 May 5;267(5606):70–72. doi: 10.1038/267070a0. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Effect of internal fluoride and phosphate on membrane currents during intracellular dialysis of nerve cells. Nature. 1975 Oct 23;257(5528):691–693. doi: 10.1038/257691a0. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Lisiewicz A. Injections of calcium ions into spinal motoneurones. J Physiol. 1972 Sep;225(2):363–390. doi: 10.1113/jphysiol.1972.sp009945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Trypsin inhibits the action of tetrodotoxin on neurones. Nature. 1977 Feb 24;265(5596):751–753. doi: 10.1038/265751a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K. Electrical excitability in the egg cell membrane of the tunicate. J Physiol. 1974 Apr;238(1):37–54. doi: 10.1113/jphysiol.1974.sp010509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Takahashi K., Tsuda K. Calcium and sodium contributions to regenerative responses in the embryonic excitable cell membrane. Science. 1972 Jun 30;176(4042):1441–1443. doi: 10.1126/science.176.4042.1441. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Membrane currents of the tunicate egg under the voltage-clamp condition. J Physiol. 1976 Jan;254(3):607–638. doi: 10.1113/jphysiol.1976.sp011249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Two components of the calcium current in the egg cell membrane of the tunicate. J Physiol. 1976 Feb;255(2):527–561. doi: 10.1113/jphysiol.1976.sp011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway E. B., Gilkey J. C., Jaffe L. F. Free calcium increases explosively in activating medaka eggs. Proc Natl Acad Sci U S A. 1977 Feb;74(2):623–627. doi: 10.1073/pnas.74.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I., Kimhi Y., Nelson P. G. Tetrodotoxin and cobalt blockade of neuroblastoma action potentials. Nat New Biol. 1973 Nov 28;246(152):124–126. doi: 10.1038/newbio246124a0. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C., Baccaglini P. I. Development of the action potential in embryo amphibian neurons in vivo. Brain Res. 1976 May 14;107(3):610–616. doi: 10.1016/0006-8993(76)90148-7. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D. Activation of sea-urchin eggs by a calcium ionophore. Proc Natl Acad Sci U S A. 1974 May;71(5):1915–1919. doi: 10.1073/pnas.71.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D., Carroll E. J., Jr, Yanagimachi R. Is calcium ionophore a universal activator for unfertilised eggs? Nature. 1974 Nov 1;252(5478):41–43. doi: 10.1038/252041a0. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. Anion interaction at the inhibitory post-synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1971 Jan;212(2):337–351. doi: 10.1113/jphysiol.1971.sp009328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Singer I., Takenaka T. Effects of internal and external ionic environment on excitability of squid giant axon. A macromolecular approach. J Gen Physiol. 1965 Jul;48(6):1095–1123. doi: 10.1085/jgp.48.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Lerman L. Role of divalent cations in excitation of squid giant axons. Am J Physiol. 1967 Dec;213(6):1465–1474. doi: 10.1152/ajplegacy.1967.213.6.1465. [DOI] [PubMed] [Google Scholar]