Abstract
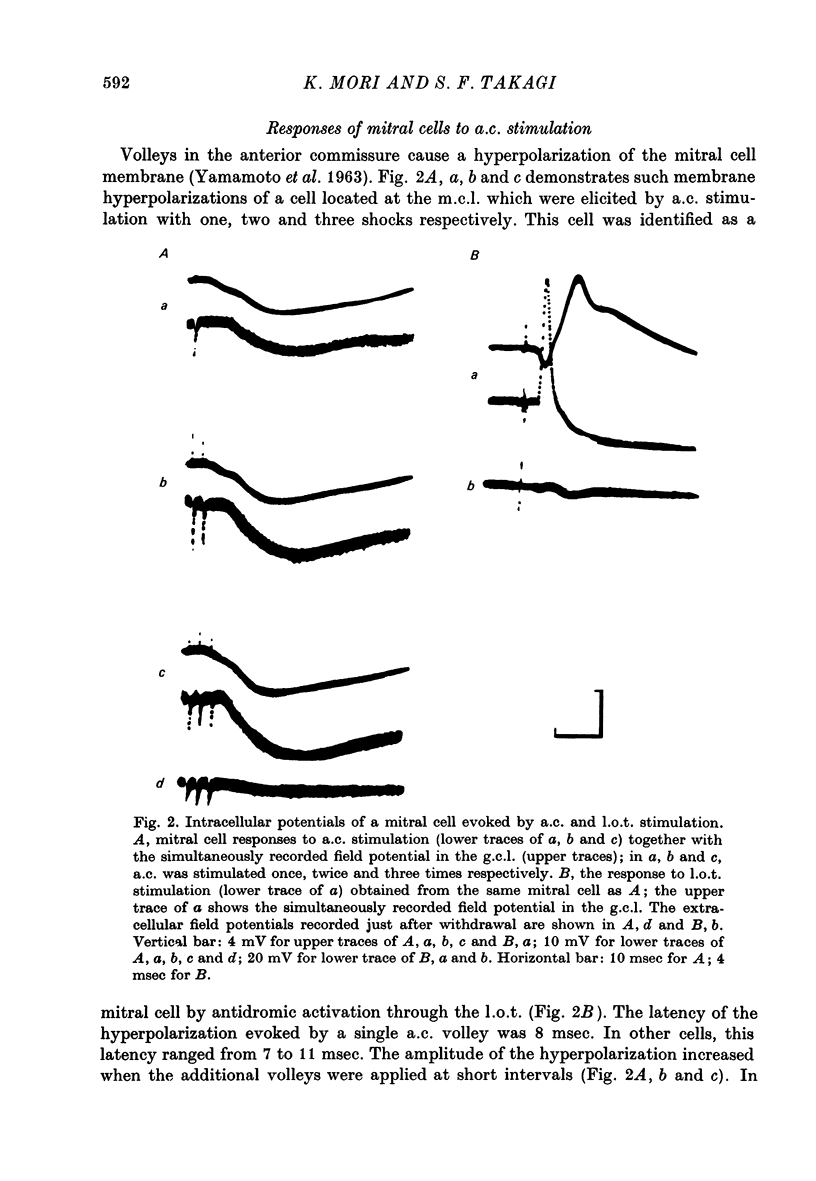
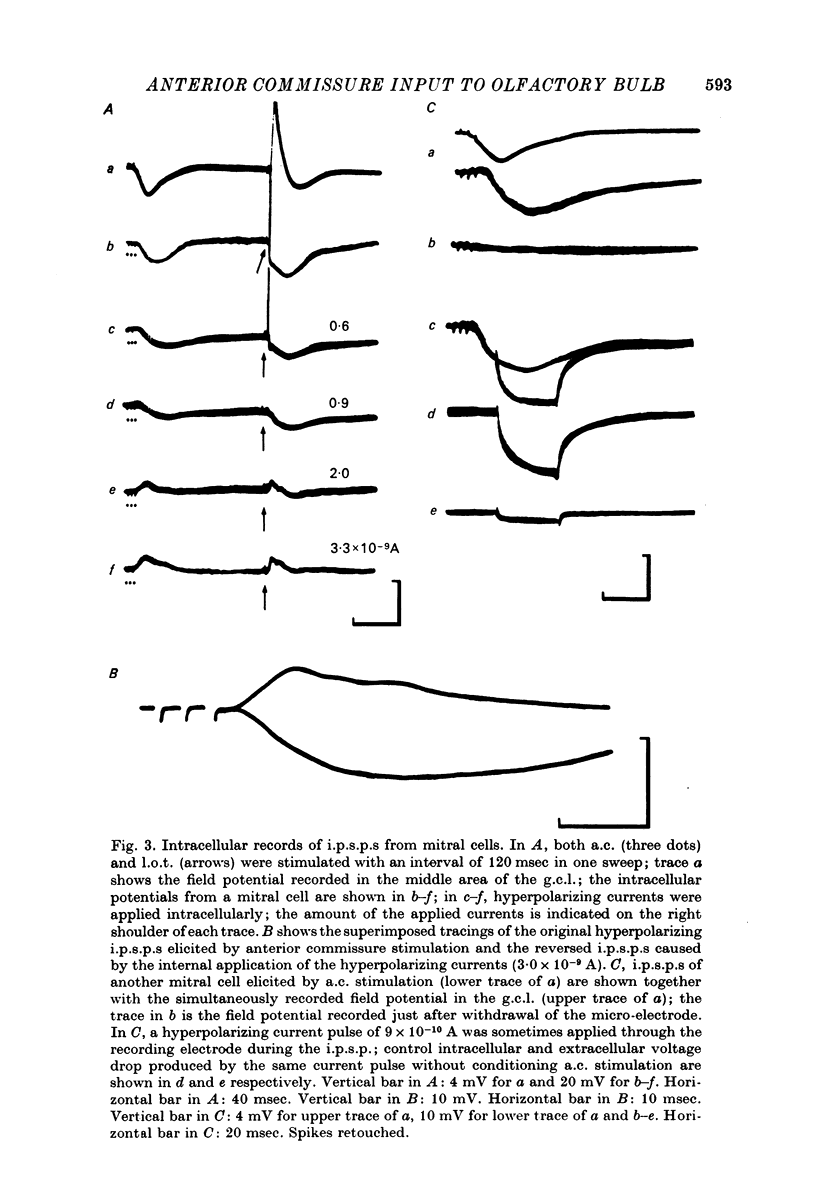
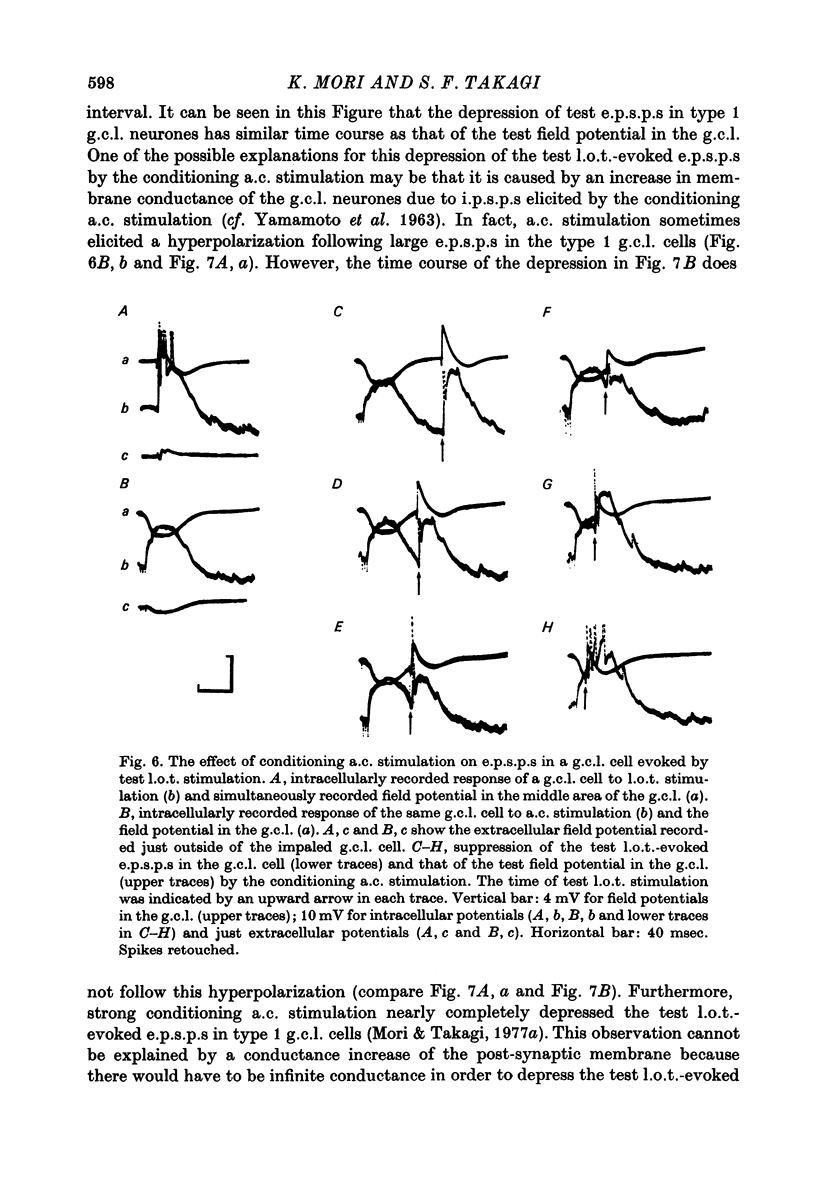
1. In the rabbit olfactory bulb, analysis has been carried out on intracellular potentials recorded from mitral cells and neurones in the granule cell layer (g.c.l. cells) in addition to the extracellular field potentials in the olfactory bulb elicited by anterior commissure (a.c.) stimulation. 2. Most mitral cell recordings showed i.p.s.p.s with latency of 7-11 msec following a.c. stimulation. These i.p.s.p.s were similar to those evoked by lateral olfactory tract (l.o.t.) stimulation in their sensitivity to internally applied current and showed asymmetrical reversal during application of the hyperpolarizing current. 3. Volleys in the a.c. elicited e.p.s.p.s in type 1 g.c.l. cells whose characteristics were in agreement with those of inhibitory interneurones inferred from the analyses of mitral cell i.p.s.p.s. It has been suggested that these type 1 g.c.l. cells may be the common inhibitory interneurones (presumably granule cells) mediating both a.c.-evoked and l.o.t.-evoked i.p.s.p.s in mitral cells. 4. Conditioning a.c. stimulation depressed the test l.o.t.-evoked i.p.s.p.s in mitral cells and test l.o.t.-evoked e.p.s.p.s in type 1 g.c.l. cells. These observations are in good agreement with the hypothesis that l.o.t.-evoked i.p.s.p.s are mainly mediated by the dendrodendritic reciprocal synapses between mitral cell dendrites and peripheral processes of granule cells. 5. The results are discussed in relation to the inhibitory mechanisms controlling mitral cell activity in the olfactory bulb.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dennis B. J., Kerr D. I. An evoked potential study of centripetal and centrifugal connections of the olfactory bulb in the cat. Brain Res. 1968 Nov;11(2):373–396. doi: 10.1016/0006-8993(68)90032-2. [DOI] [PubMed] [Google Scholar]
- KERR D. I., HAGBARTH K. E. An investigation of olfactory centrifugal fiber system. J Neurophysiol. 1955 Jul;18(4):362–374. doi: 10.1152/jn.1955.18.4.362. [DOI] [PubMed] [Google Scholar]
- KERR D. I. Properties of the olfactory efferent system. Aust J Exp Biol Med Sci. 1960 Feb;38:29–36. doi: 10.1038/icb.1960.4. [DOI] [PubMed] [Google Scholar]
- Mori K., Takagi S. F. An intracellular study of dendrodendritic inhibitory synapses on mitral cells in the rabbit olfactory bulb. J Physiol. 1978 Jun;279:569–588. doi: 10.1113/jphysiol.1978.sp012362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A. Inhibitory mechanisms in the rabbit olfactory bulb: dendrodendritic mechanisms. Brain Res. 1969 Jun;14(1):157–172. doi: 10.1016/0006-8993(69)90037-7. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Recurrent excitatory pathways of anterior commissure and mitral cell axons in the olfactory bulb. Brain Res. 1970 May 4;19(3):491–493. doi: 10.1016/0006-8993(70)90392-6. [DOI] [PubMed] [Google Scholar]
- OCHI J. Olfactory bulb response to antidromic olfactory tract stimulation in the rabbit. Jpn J Physiol. 1963 Apr 15;13:113–128. doi: 10.2170/jjphysiol.13.113. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G., POWELL T. P., SHEPHERD G. M. RESPONSES OF MITRAL CELLS TO STIMULATION OF THE LATERAL OLFACTORY TRACT IN THE RABBIT. J Physiol. 1963 Aug;168:65–88. doi: 10.1113/jphysiol.1963.sp007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. L., Powell T. P. An electron-microscopic study of the termination of the afferent fibres to the olfactory bulb from the cerebral hemisphere. J Cell Sci. 1970 Jul;7(1):157–187. doi: 10.1242/jcs.7.1.157. [DOI] [PubMed] [Google Scholar]
- Price J. L., Powell T. P. The mitral and short axon cells of the olfactory bulb. J Cell Sci. 1970 Nov;7(3):631–651. doi: 10.1242/jcs.7.3.631. [DOI] [PubMed] [Google Scholar]
- Price J. L., Powell T. P. The synaptology of the granule cells of the olfactory bulb. J Cell Sci. 1970 Jul;7(1):125–155. doi: 10.1242/jcs.7.1.125. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M., Reese T. S., Brightman M. W. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp Neurol. 1966 Jan;14(1):44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968 Nov;31(6):884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- Shepherd G. M. Synaptic organization of the mammalian olfactory bulb. Physiol Rev. 1972 Oct;52(4):864–917. doi: 10.1152/physrev.1972.52.4.864. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO C., YAMAMOTO T., IWAMA K. The inhibitory systems in the olfactory bulb studied by intracellular recording. J Neurophysiol. 1963 May;26:403–415. doi: 10.1152/jn.1963.26.3.403. [DOI] [PubMed] [Google Scholar]
- von BAUMGARTEN, GREEN J. D., MANCIA M. Recurrent inhibition in the olfactory bulb. II. Effects of antidromic stimulation of commissural fibers. J Neurophysiol. 1962 Jul;25:489–500. doi: 10.1152/jn.1962.25.4.489. [DOI] [PubMed] [Google Scholar]


