Abstract
Streptococcus pneumoniae is a major cause of disease, especially in developing countries, and cost-effective alternatives to the currently licensed vaccines are needed. We constructed DNA vaccines based on pneumococcal surface protein A (PspA), an antigen shown to induce protection against pneumococcal bacteremia. PspA fragments can be divided into three families, which can be subdivided into six clades, on the basis of PspA amino acid sequence divergence (S. K. Hollingshead, R. Becker, and D. E. Briles, Infect. Immun. 68:5889-5900, 2000). Since most clinical isolates belong to family 1 or family 2, PspA fragments from members of both of these families were analyzed. Vectors encoding the complete N-terminal regions of PspAs elicited significant humoral responses, and cross-reactivity was mainly restricted to the same family. DNA vaccines encoding fusions between PspA fragments from family 1 and family 2 were also constructed and were able to broaden the cross-reactivity, with induction of antibodies that showed reactions with members of both families. At least for the pneumococcal strains tested, the cross-reactivity of antibodies was not reflected in cross-protection. Animals immunized with DNA vaccines expressing the complete N-terminal regions of PspA fragments were protected only against intraperitoneal challenge with a strain expressing PspA from the same clade.
Streptococcus pneumoniae is a major cause of diseases, including pneumonia, otitis, meningitis, and bacteremia. About 1 million children die of pneumococcal disease every year, mostly in developing countries, but elderly persons are also at risk in the developed world. Conjugated vaccines composed of 7 to 11 selected polysaccharides bound to a protein carrier were recently licensed. Although these vaccines are able to elicit protective T-cell-dependent immune responses, their reactivities are restricted to the polysaccharide serotypes included in the formulations (17). Furthermore, the cost of these vaccines is a major obstacle for their widespread use in developing countries.
An alternative to conjugated vaccines could be the use of protective protein antigens that are expressed in the great majority of pneumococcal strains. Pneumococcal surface protein A (PspA) is one of the most promising vaccine candidates; passive immunization with anti-PspA antibodies or active immunization with purified full-length or truncated PspA has been shown to protect mice against infection with pneumococci of different serotypes (10, 14). Recently, immunization of humans with a single recombinant fragment of PspA was shown to induce antibodies that passively protect mice against challenge with different S. pneumoniae strains (4). PspA has been shown to be involved in iron limitation overcome through binding to lactoferrin (7) and also has been shown to inhibit complement activation (15). One of the concerns with the use of this protein as a vaccine is the structural and antigenic variability among different strains (5). PspA is composed of five domains: a signal peptide, an α-helical domain, a proline-rich region, a choline binding domain, and a C-terminal tail (18). Antigenic variability has been mapped to the highly charged α-helical N-terminal region of PspA (11). Hollingshead et al. (8) proposed a classification for PspA based on divergence of the amino acid sequence located just before the proline-rich region, designated the clade-defining region (CDR). This classification divides PspA into three families: family 1, composed of clades 1 and 2; family 2, composed of clades 3, 4, and 5; and family 3, rarely isolated and composed of clade 6. Since cross-reactivity between families is restricted, they propose that PspA molecules from families 1 and 2 should be included in a vaccine formulation.
Genetic immunization with the region encoding the α-helical domain of PspA from strain Rx1 (clade 2) was recently shown to elicit protective immunity against S. pneumoniae (2). In this work, we cloned different PspA fragments belonging to clades 1, 2, 3, and 4 into DNA vaccine vectors and analyzed the cross-reactivity of anti-PspA antibodies, as well as the cross-protection against intraperitoneal challenge with S. pneumoniae induced by immunization of mice with each of the constructs.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All cloning procedures were performed in Escherichia coli DH5α grown in Luria-Bertani medium supplemented with ampicillin (100 μg/ml). Strains of S. pneumoniae isolated in Brazil from invasive infections were obtained from Instituto Adolfo Lutz (São Paulo, Brazil); these strains were seeded into blood agar tubes and grown overnight at 37°C. Cultures were then grown in Todd-Hewitt liquid medium containing 0.5% yeast extract (Difco, Detroit, Mich.) and incubated with gentle shaking at 37°C. Cultures in the exponential phase were frozen and stored at −80°C in Todd-Hewitt liquid medium containing 0.5% yeast extract and 10% glycerol. The viability of bacterial stocks was analyzed prior to challenge.
DNA vaccine vectors.
The vectors used for nucleic acid immunization were based on the pTARGET expression vector (Promega, Madison, Wis.), in which the gene is expressed under human cytomegalovirus immediate early promoter-enhancer control. Different DNA fragments encoding PspAs from of clades 1, 2, 3, and 4 were cloned by PCR from genomic DNA into pTARGET. Table 1 shows the strains from which fragments were cloned, the PspA domains present in each fragment, and the primers used for cloning. In all cases, the ACC Kozak consensus sequence for vertebrate mRNAs was added before the ATG start codon (9). The DNA sequences were analyzed with an ABI Prism DNA sequencer (PE Applied Biosystems, Foster City, Calif.).
TABLE 1.
PspA fragments
| Vector | Strain | PspA domain(s) | Primers |
|---|---|---|---|
| pTG-pspA1 | St 435/96 (clade 1) | α-helix, CDR, proline rich | 5′TAG CTC GAG ACC ATG ATC TTA GGG GCT GGT TT3′ (forward), 5′TAG TTA TCT AGA TGG TTG TGG TGC TGA AG3′ (reverse) |
| pTG-pspA2 | St 371/00 (clade 2) | CDR | 5′TAG TCT AGA ACC ATG CTC AAA GAG ATT GAT GAG TCT3′ (forward), 5′TAG GGT ACC TTA TTC TGG CTC ATT AAC TGC TTT3′ (reverse) |
| pTG-pspA3 | St 259/98 (clade 3) | α-helix, CDR, proline rich | 5′ TAG CTC GAG ACC ATG ATC TTA GGG GCT GGT TT3′ (forward), 5′ TAG TTA TCT AGA TTT TGG TGC AGG AGC TGG3′ (reverse) |
| pTG-pspA4 | St 255/00 (clade 4) | CDR | 5′TAG TCT AGA ACC ATG TTA GAA AAA GCA GAA GCT GAA3′ (forward), 5′ TAG GGTACC TTA TTC TTC ATC TCC ATC AGG G3′ (reverse) |
| pTG-pspA1/4 | Clade 1 (α-helix, CDR, proline rich) + clade 4 (CDR) | ||
| pTG-pspA3/2 | Clade 3 (α-helix, CDR, proline rich) + clade 2 (CDR) |
Antigen expression in transfected mammalian cells.
In vitro expression of PspA was analyzed in transiently transfected BHK-21 (baby hamster kidney) cells by using Lipofectamine (Life Technologies, Rockville, Md.) according to the manufacturer's instructions. Expression was characterized by Western blotting of total extracts of BHK-21 cells 1 day after transfection by using standard procedures (13). Approximately 30 μg of protein extract was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred onto a nitrocellulose membrane. Mouse polyclonal anti-PspA1 antiserum (1:1,000) was developed against PspA purified from St 435/96 (clade 1) and anti-PspA3 antiserum (1:1,000) was developed against PspA purified from St 259/98 (clade 3) by using the choline chloride wash method described by Briles et al. (3). Horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (1:1,000; Sigma Chemical Co., St. Louis, Mo.) was used, and detection was performed by using a chemiluminescent ECL kit (Amersham-Pharmacia Biotech, Little Chalfont, England).
Immunization of mice.
Plasmid DNA was purified from E. coli DH5α by anion-exchange chromatography with a Concert High-Purity Maxiprep system (Life Technologies). Female BALB/c mice from Instituto Butantan (São Paulo, Brazil) were inoculated in each muscle tibialis anterior as previously described (6) with 50 μl of 10 μM cardiotoxin (Latoxan, Valence, France) and then after 5 days with 25 μg of plasmid DNA in phosphate-buffered saline (PBS) (50 μl). The mice received a booster dose under the same conditions after 3 weeks and were bled from the retroorbital plexus 3 and 6 weeks after priming for detection of anti-PspA serum antibodies. For immunization with purified proteins, mice were injected intraperitoneally with 10 μg of protein with Al(OH)3 as the adjuvant and were boosted under the same conditions after 3 weeks.
Determination of anti-PspA serum antibodies and isotyping.
Antibodies against PspA were detected by an enzyme-linked immunosorbent assay (ELISA). Briefly, Polysorp 96-well plates (Nunc, Roskilde, Denmark) were coated with PspA (1 μg/ml) isolated by choline chloride washing from S. pneumoniae strains expressing PspA belonging to different clades, as previously described (3). The plates were washed three times with PBS containing 0.1% Tween 20 and blocked with 10% nonfat dry milk in PBS. The plates were then incubated with serial dilutions of sera from the immunized mice in PBS-1% bovine serum albumin at 37°C for 1 h. The plates were washed as described above and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (1:2,000) (Sigma) in PBS-1% bovine serum albumin at 37°C for 1 h. Following the washes, antibodies were detected by adding OPD substrate (0.04% o-phenylenediamine in citrate-phosphate buffer [pH 5] containing 0.01% H2O2). After color development (10 min), the reaction was interrupted with 1.25 M H2SO4, and the A492 was determined. The reciprocal titer was considered the last dilution of serum that registered an optical density of 0.10. IgG1 and IgG2a were also detected by the ELISA by using mouse monoclonal antibody isotyping reagents (Sigma).
Intraperitoneal challenge.
Mice were challenged intraperitoneally with 2 × 106 CFU of S. pneumoniae, and survival of mice was analyzed daily for 21 days. Inactive sick animals were euthanized. Differences in the overall survival rates between groups were analyzed by the Fisher exact test.
Nucleotide sequence accession numbers.
The pspA sequences have been deposited in the GenBank database under accession no. AY082387 to AY082390.
RESULTS
Cloning of pspA fragments from clades 1, 2, 3, and 4.
pspA fragments from different S. pneumoniae strains (clades 1, 2, 3, and 4) were cloned into the DNA vaccine vector pTARGET, generating pTG-pspA1, pTG-pspA2, pTG-pspA3, and pTG-pspA4. As shown in Table 1, pTG-pspA1 and pTG-pspA3 encode the N-terminal α-helix (including the CDR) and the proline-rich region, while pTG-pspA2 and pTG-pspA4 encode only the CDR. The predicted amino acid sequences were analyzed, and the cloned fragments were divided into the different clades according to the classification proposed by Hollingshead et al. (8). DNA vaccines encoding fusions between clade 1 and clade 4 fragments (pTG-pspA1/4) and between clade 3 and clade 2 fragments (pTG-pspA3/2) were also constructed for expression of PspA fusions composed of fragments of both family 1 and family 2.
Expression of PspA in mammalian cells.
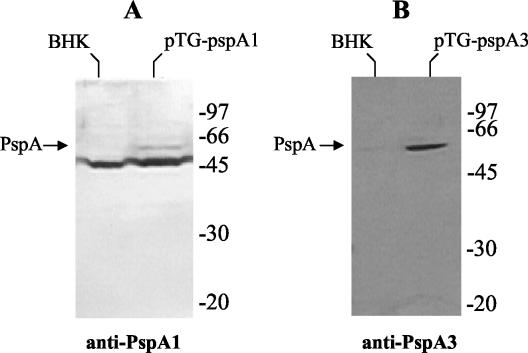
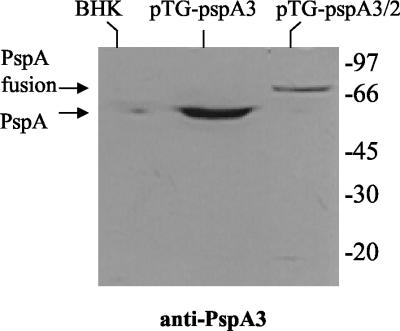
DNA vaccine vectors pTG-pspA1, pTG-pspA2, pTG-pspA3, and pTG-pspA4 were transfected into BHK-21 cells, and expression of the PspA fragments was analyzed 1 day after transfection by Western blotting with antisera raised against native PspA purified from a pneumococcal strain expressing clade 1 PspA (anti-PspA1) and one expressing clade 3 PspA (anti-PspA3). Expression of PspA by pTG-pspA1 was analyzed with both sera but was detected only with anti-PspA1 (Fig. 1A). Expression of PspA by pTG-pspA3 was also analyzed with both sera and was detected only with anti-PspA3 (Fig. 1B). These results indicate that the antisera used show little cross-reactivity between families, since clade 1 belongs to family 1 and clade 3 to family 2. Expression of the CDR from PspA2 was not observed in cells transfected with pTG-pspA2, and expression of PspA4 from pTG-pspA4 was not detected with any of the antisera (data not shown). Expression of the PspA fusion protein by pTG-pspA1/4 was also not detected by Western blotting (data not shown), which could indicate a lower level of expression than the level observed with pTG-pspA1. On the other hand, expression of the fusion protein by pTG-pspA3/2 was observed when anti-PspA3 (Fig. 2) was used, although the levels were lower than the levels observed with pTG-pspA3.
FIG. 1.
Transient expression of PspA by the DNA vaccine vectors in BHK-21 cells. Total extracts of BHK-21 cells transformed or not transformed with the vectors were analyzed by Western blotting by using anti-PspA1 (A) or anti-PspA3 (B) antiserum. The positions of standard molecular weight markers are indicated on the right.
FIG. 2.
Transient expression of PspA fusion by the DNA vaccine vector in BHK-21 cells. Total extracts of BHK-21 cells transformed or not transformed with the vectors were analyzed by Western blotting by using anti-PspA3 antiserum. The positions of standard molecular weight markers are indicated on the right.
Cross-reactivities of antibodies induced by DNA vaccination.
BALB/c mice were immunized with the DNA vaccine vectors described above and received a booster dose after 3 weeks. Serum antibodies against the purified proteins were detected 6 weeks after priming by the ELISA. Table 2 shows the anti-PspA antibody titers for sera of mice immunized with each of the DNA vaccines. Mice immunized with pTG-pspA1 showed induction of antibodies that reacted with PspA from clade 1 and clade 2 (both family 1), while sera from animals immunized with pTG-pspA3 reacted with PspA from clade 3 and clade 4 (both family 2). These results confirmed the data obtained previously that indicated that cross-reactivity between PspAs occurs mainly within the different families. Furthermore, immunization with pTG-pspA2 and pTG-pspA4 did not induce reactive antibodies to any of the PspAs, even from the same clade. Interestingly, immunization with the vectors expressing the fusions between clades 1 and 4 and between clades 3 and 2 led to induction of antibodies that reacted with both family 1 and family 2 PspAs, indicating that these fusions are indeed able to enhance the cross-reactivity of the antibodies induced by DNA vaccination.
TABLE 2.
Antibody titers induced by immunization with DNA vaccine vectors
| Immunization vectora | Antibody titers with the following coating antigenb:
|
|||
|---|---|---|---|---|
| Family 1
|
Family 2
|
|||
| PspA1 (St 435/96) | PspA2 (St 371/00) | PspA3 (St 259/98) | PspA4 (St 255/00) | |
| Saline | 1:160 | 1:160 | 1:160 | 1:160 |
| pTG-pspA1 | 1:10,240 | 1:1,280 | 1:40 | 1:320 |
| pTG-pspA2 | <1:20 | 1:20 | 1:40 | 1:20 |
| pTG-pspA3 | 1:20 | 1:20 | 1:1,280 | 1:1,280 |
| pTG-pspA4 | 1:20 | 1:20 | 1:80 | 1:160 |
| pTG-pspA1/4 | 1:10,240 | 1:640 | 1:160 | 1:2,560 |
| pTG-pspA3/2 | 1:20 | 1:640 | 1:10,240 | 1:5,120 |
Sera were obtained from mice that were immunized with the DNA vaccine vectors or injected with only saline.
ELISA plates were coated with PspAs purified from the pneumococcal strains.
IgG1/IgG2a ratio of antibodies induced by DNA vaccination.
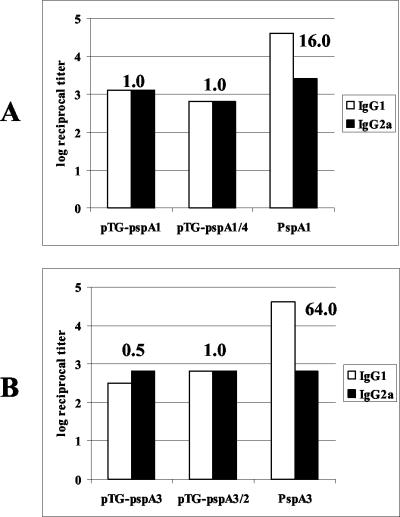
Antibodies induced by DNA vaccination were isotyped by ELISA. Figure 3 shows IgG1 and IgG2a antibody titers induced by pTG-pspA1 and pTG-pspA1/4 (Fig. 3A) or by pTG-pspA3 and pTG-pspA3/2 (Fig. 3B) compared to the titers induced by immunization with PspA purified from pneumococci and adsorbed with Al(OH)3. The antibody titers induced by DNA immunization showed IgG1/IgG2a ratios between 0.5 and 1.0, while the antibody titers induced by immunization with protein showed preferential induction of IgG1, with IgG1/IgG2a ratios between 16.0 and 64.0.
FIG. 3.
Induction of IgG1 and IgG2a by immunization with the DNA vaccine vectors. BALB/c mice were immunized with the DNA vaccine vectors or purified proteins, sera were collected 6 weeks after priming, and anti-PspA IgG1 and IgG2a were detected by ELISA in plates coated with PspA1 (A) or PspA3 (B). The log of the anti-PspA reciprocal antibody titer is shown. The numbers above the bars are the IgG1/IgG2a reciprocal titer ratios.
Intraperitoneal challenge.
Immunized mice were challenged intraperitoneally with S. pneumoniae strains 6 weeks after priming, and survival was scored for 21 days. Table 3 shows the data for mice challenged with St 491/00 (capsular serotype 6B, PspA clade 1 in family 1). Of the animals injected with DNA vaccines, only those immunized with pTG-pspA1 (P = 0.05) and pTG-pspA1/4 (P = 0.02) showed significantly higher survival than nonimmunized mice. Immunization with purified protein (PspA 491/00) also induced significant protection (P < 0.001). Table 4 shows the survival of mice after intraperitoneal challenge with St 472/96 (capsular serotype 6B, PspA clade 4 in family 2). In this case, only purified protein (PspA 472/96) resulted in significant protection (P = 0.002), with all animals surviving the challenge. None of the DNA vaccine vectors was able to induce significant protection.
TABLE 3.
Survival of mice challenged with St 491/00 (serotype 6B, clade 1 in family 1)
| Immunization vector | No. alive/total no. | % Survival |
|---|---|---|
| Saline | 3/18 | 17 |
| pTG-pspA1 | 6/12 | 50a |
| pTG-pspA3 | 2/12 | 17 |
| pTG-pspA1/4 | 7/12 | 58a |
| pTG-pspA3/2 | 2/12 | 17 |
| PspA 491/00 | 13/18 | 72a |
The value is significantly different from the value for nonimmunized mice (P ≤ 0.05).
TABLE 4.
Survival of mice challenged with St 472/96 (serotype 6B, clade 4 in family 2)
| Immunization vector | No. alive/total no. | % Survival |
|---|---|---|
| Saline | 8/18 | 44 |
| pTG-pspA1 | 9/18 | 50 |
| pTG-pspA3 | 7/12 | 58 |
| pTG-pspA1/4 | 5/11 | 45 |
| pTG-pspA3/2 | 8/12 | 67 |
| PspA 472/96 | 11/11 | 100a |
The value is significantly different from the value for nonimmunized mice (P ≤ 0.05).
DISCUSSION
Vaccines based on proteins are an interesting alternative to conjugated vaccines for use against S. pneumoniae. PspA is one of the most promising antigens, but it shows considerable diversity among clinical isolates. A recent analysis of amino acid sequence divergence (8) divided PspA into three families. In the present work we analyzed DNA vaccines encoding different PspA fragments from family 1 and family 2.
DNA vaccines containing pspA fragments coding for the complete N-terminal α-helix plus the proline-rich region were expressed in mammalian cells. Immunization of mice with these vaccines induced antibodies that reacted in ELISA only with PspAs from the homologous family. On the other hand, expression of the fragments encoding only the CDR, which was shown to contain the most important antigenic epitopes, was not observed. These results could have been due either to poor expression of the antigen or to a lack of cross-reactivity with the sera used in the Western blot experiments. Furthermore, immunization of mice with these constructs did not induce antibodies to any of the PspAs, not even from the same clade, which reinforces the idea that vaccines encoding only the CDR of PspA might be poorly expressed. DNA vaccines encoding fusions of PspA fragments were also constructed so that the antigens were composed of portions of PspAs from both family 1 and family 2. Although these constructs showed lower expression or could not be detected at all in Western blot experiments, they could elicit antibodies that reacted with PspAs from both families, showing that this could be an interesting approach for the development of vaccines with broader cross-reactivity. Furthermore, balanced IgG1/IgG2a ratios ranging from 0.5 to 1.0 were induced by DNA vaccination, which contrasts with the preferential induction of IgG1 by immunization with purified protein in the presence of alum. Since it was recently shown that the augmentation of antibody-mediated opsonization after intranasal immunization of mice with PspA and interleukin-12 was due mainly to IgG2a (1), we propose that DNA vaccination could be an interesting approach for the development of vaccines against S. pneumoniae.
The challenge experiments described in this paper showed that cross-reactivity of antibodies induced by DNA vaccination does not correlate with cross-protection against intraperitoneal challenge. As expected, mice challenged with a pneumococcal strain that has a clade 1 PspA (family 1) were protected when they were immunized with the DNA vaccines expressing the whole N-terminal region of a clade 1 PspA (pTG-pspA1) or its fusion with the CDR of a clade 4 PspA (pTG-pspA1/4). Expression of a clade 3 PspA or a clade 3-clade 2 PspA fusion did not elicit protection. On the other hand, mice challenged with a pneumococcal strain carrying a clade 4 PspA (family 2) were not protected by the DNA vaccines expressing the whole N-terminal region of either a clade 1 (family 1) or a clade 3 (family 2) PspA, even though pTG-pspA3 did elicit antibodies that cross-reacted with PspA4. DNA vaccines encoding PspA fusions also did not elicit significant protection, even with expression of the CDR of a clade 4 PspA. Protection was thus restricted to immunization with a DNA vaccine expressing a complete N-terminal PspA fragment belonging to the same clade as the strain used for the challenge. The induction of cross-reactive antibodies detected by ELISA does not always correlate completely with functional cross-reactivity. Analysis of serum samples from individuals immunized with pneumococcal vaccines showed that cross-reactive antibodies induced against capsular polysaccharide detected by ELISA do not entirely reflect the opsonophagocytic function of the antibodies (12, 16).
Nevertheless, Briles et al. (4) showed that immunization of humans with recombinant PspA from strain Rx1 (clade 2) could induce passive protection of mice against fatal infection with S. pneumoniae strains expressing either family 1 or family 2 PspAs. The use of DNA vaccination rather than protein vaccination could explain these discrepancies, since the induction of antibody titers by DNA vaccination was lower than the induction of antibody titers by protein immunization. Virulence variation between strains expressing PspA belonging to the same clade could significantly influence challenge experiments and could also explain such differences. Furthermore, it is important to point out that in their study Briles et al. (4) used human sera, while our work was performed with mice. Our results thus confirm the notion that cross-reactivity of PspA is restricted to the same family; however, at least for the pneumococcal strains tested, protection against intraperitoneal challenge was restricted primarily to the same clade.
Acknowledgments
We thank Susan K Hollingshead for fruitful discussions. We also thank Marisa G. Trevelin, Solange R. Silva, and Sebastiana V. Oliveira for technical assistance and Fátima A. M. Oliveira for secretarial assistance.
This work was supported by FAPESP grant 99/05202-1, by Fundação Butantan, and by CNPq grant 520580/00-1 (Brazil).
Editor: E. I. Tuomanen
REFERENCES
- 1.Arulanadam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosarge, J. R., J. M. Watt, O. McDaniel, E. Swiatlo, and L. S. McDaniel. 2001. Genetic immunization with the region encoding the α-helical domain of PspA elicits protective immunity against Streptococcus pneumoniae. Infect. Immun. 69:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briles, D. E., J. D. King, M. A. Gray, L. S. McDaniel, E. Swiatlo, and K. A. Benton. 1996. PspA, a protection-eliciting protein: immunogenicity of isolated native PspA in mice. Vaccine 14:858-867. [DOI] [PubMed] [Google Scholar]
- 4.Briles, D. E., S. K. Hollingshead, J. King, A. Swift, P. A. Braun, M. K. Park, L. M. Ferguson, M. H. Nahm, and G. S. Nabors. 2000. Immunizations of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694-1701. [DOI] [PubMed] [Google Scholar]
- 5.Crain, M. J., W. D. Waltman II, J. S. Turner, J. Yother, D. F. Talkington, L. S. McDaniel, B. M. Gray, and D. E. Briles. 1990. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58:3293-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, H. L., M.-L. Michel, and R. G. Whalen. 1993. DNA based immunization for hepatitis B induces continuous secretion of antigen and high levels of circulating antibody. Hum. Mol. Genet. 2:1847-1851. [DOI] [PubMed] [Google Scholar]
- 7.Hammerschmidt, S., G. Bethe, P. H. Remane, and G. S. Chhatwal. 1999. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun. 67:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollingshead, S. K., R. Becker, and D. E. Briles. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozak, M. 1987. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 20:8125-8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDaniel, L. S., J. S. Sheffield, P. Delucchi, and D. E. Briles. 1991. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect. Immun. 59:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDaniel, L. S., B. A. Ralph, D. O. McDaniel, and D. E. Briles. 1994. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb. Pathog. 17:323-337. [DOI] [PubMed] [Google Scholar]
- 12.Nahm, M. H., J. V. Olander, and M. Magyarlaki. 1997. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J. Infect. Dis. 176:698-703. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 14.Tart, R. C., L. S. McDaniel, B. A. Ralph, and D. E. Briles. 1996. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J. Infect. Dis. 173:380-386. [DOI] [PubMed] [Google Scholar]
- 15.Tu, A. T., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vakevainen, M., C. Eklund, J. Eskola, and H. Kathy. 2001. Cross-reactivity of antibodies to type 6B and 6A polysaccharides of Streptococcus pneumoniae, evoked by pneumococcal conjugate vaccines, in infants. J. Infect. Dis. 184:789-793. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. 1999. Pneumococcal vaccines. Wkly. Epidemiol. Rec. 74:177-184.10437429 [Google Scholar]
- 18.Yother, J., and J. White. 1994. Novel surface attachment of the Streptococcus pneumoniae protein PspA. J. Bacteriol. 176:2976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]