Abstract
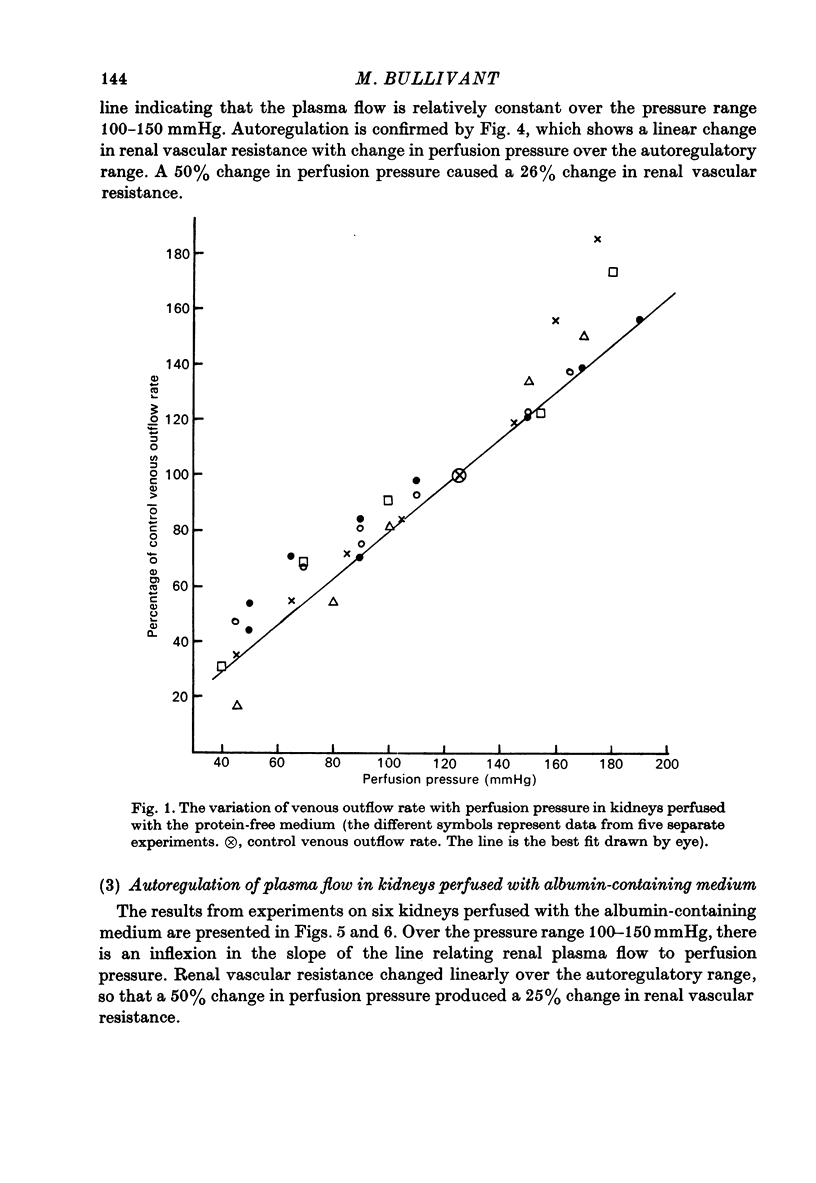
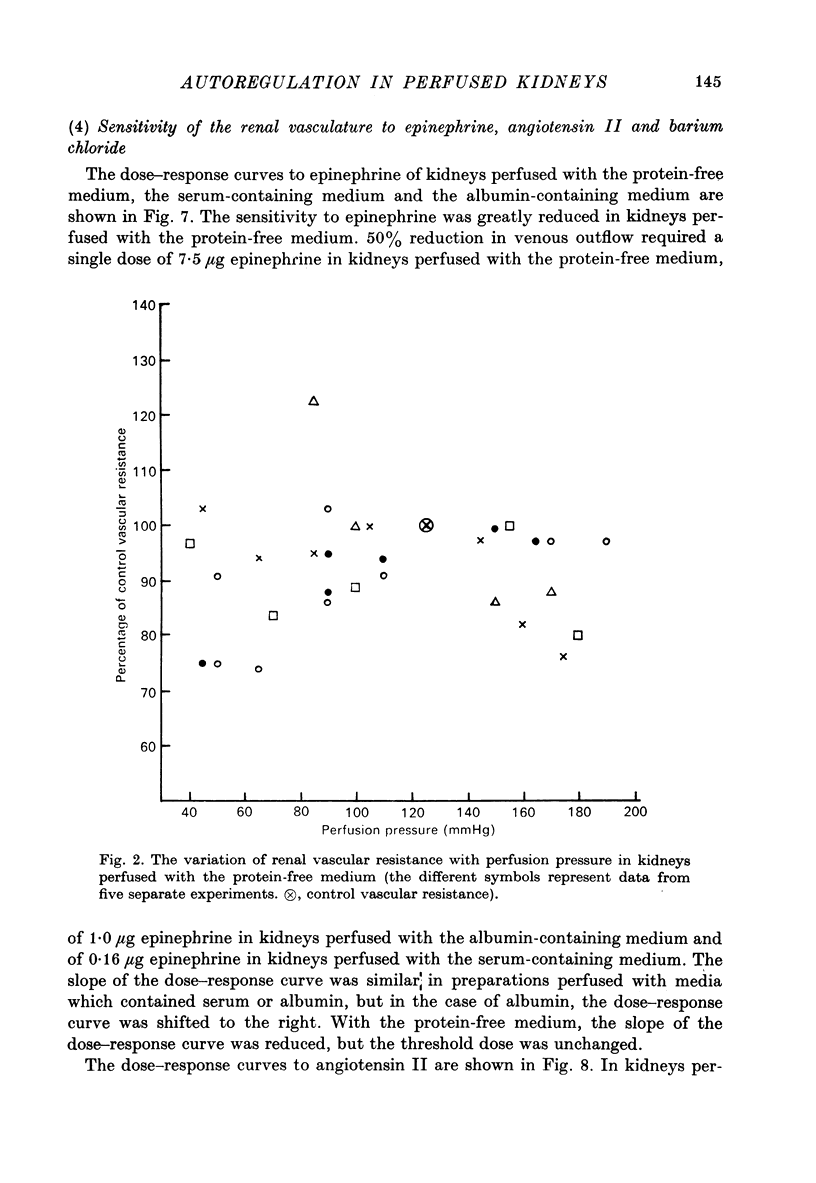
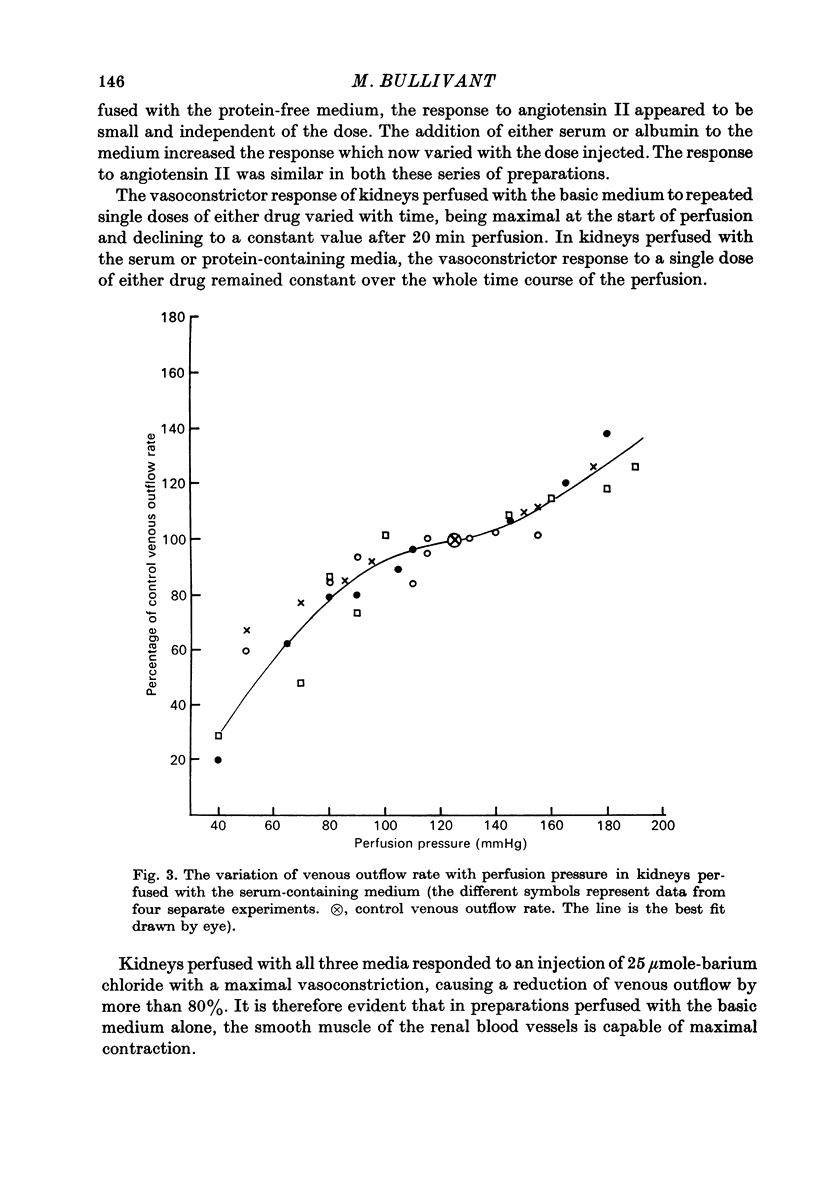
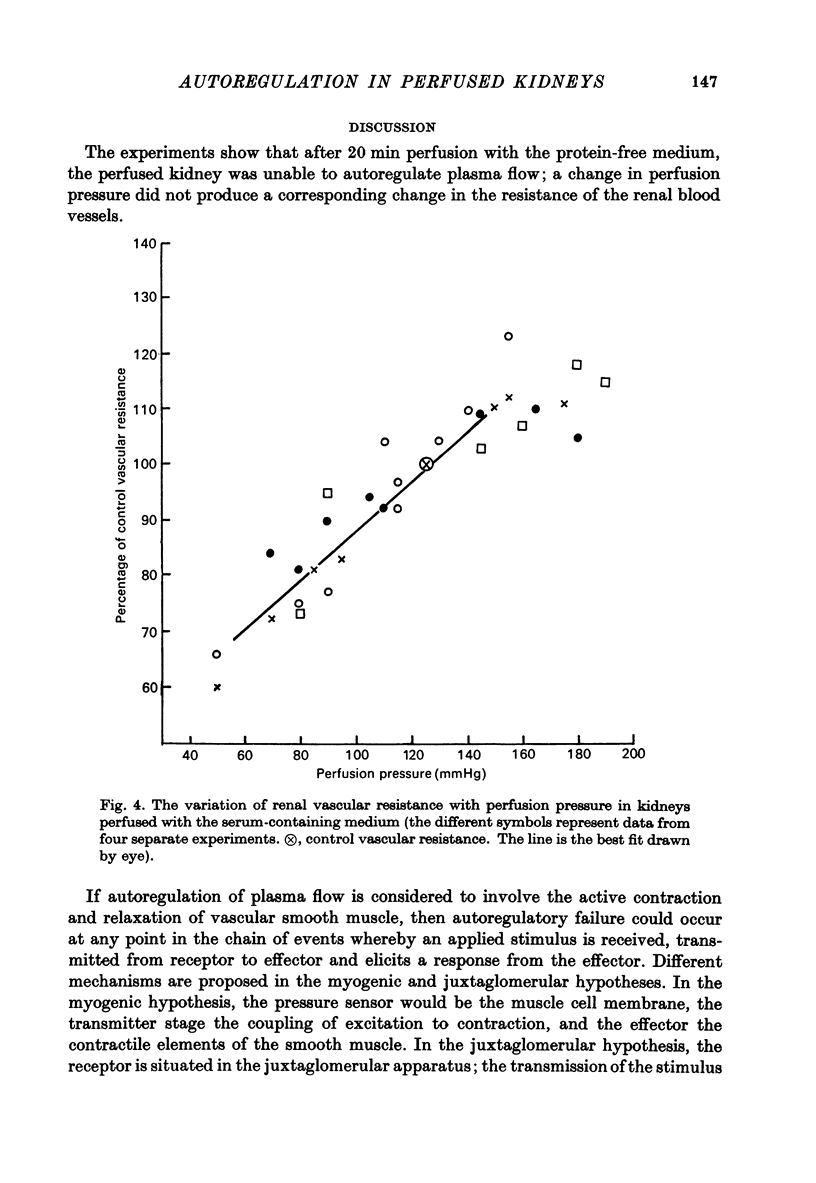
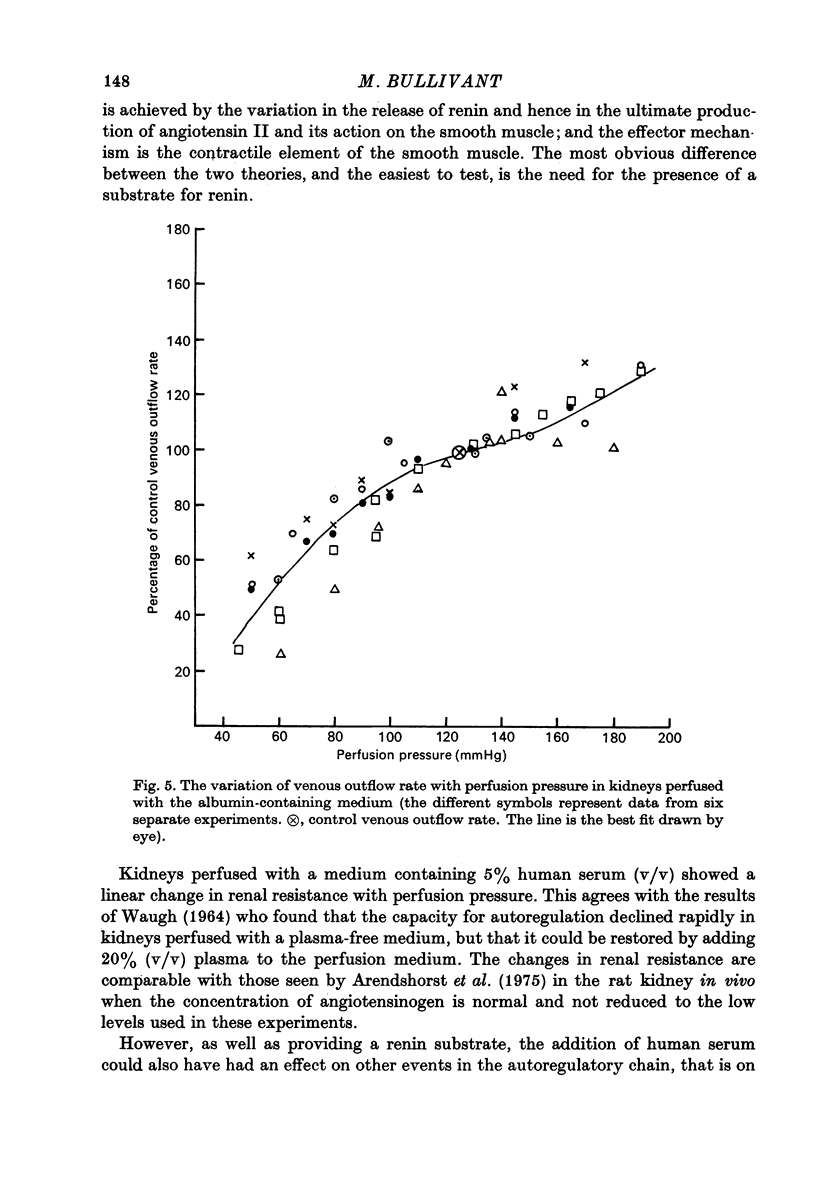
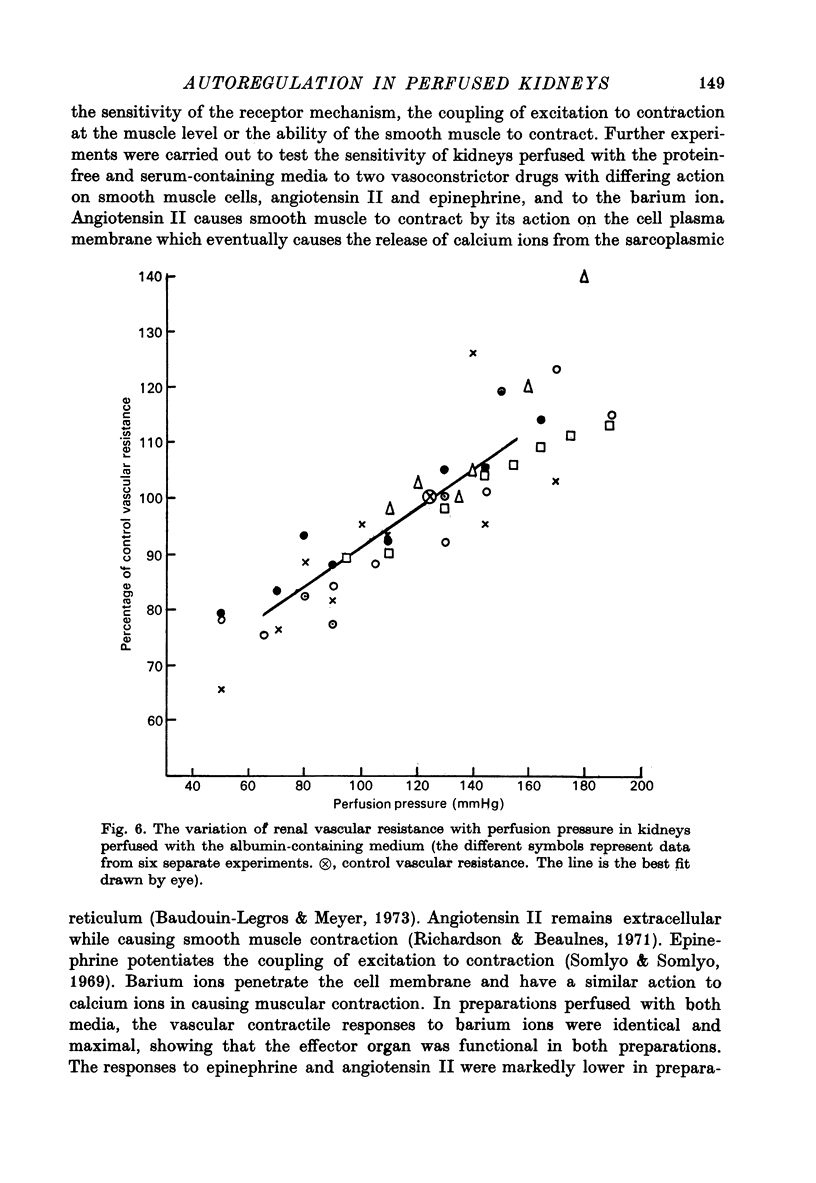
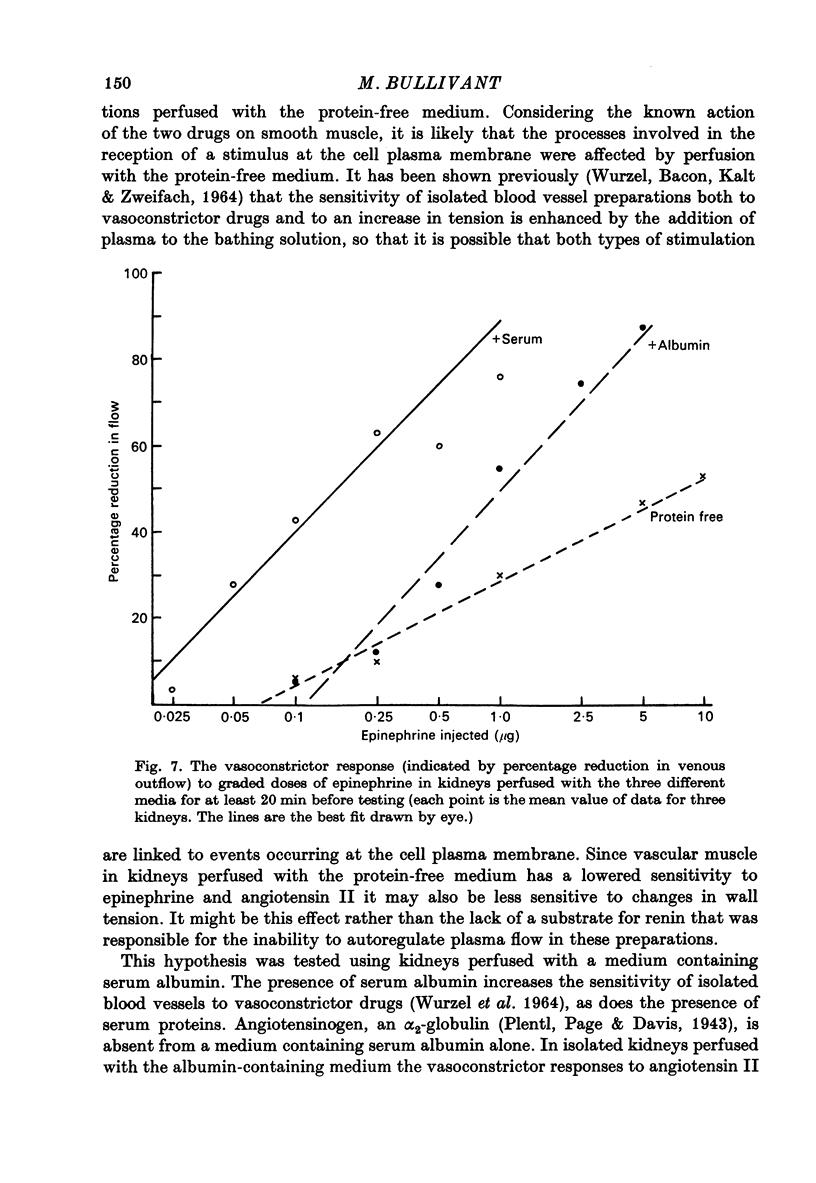
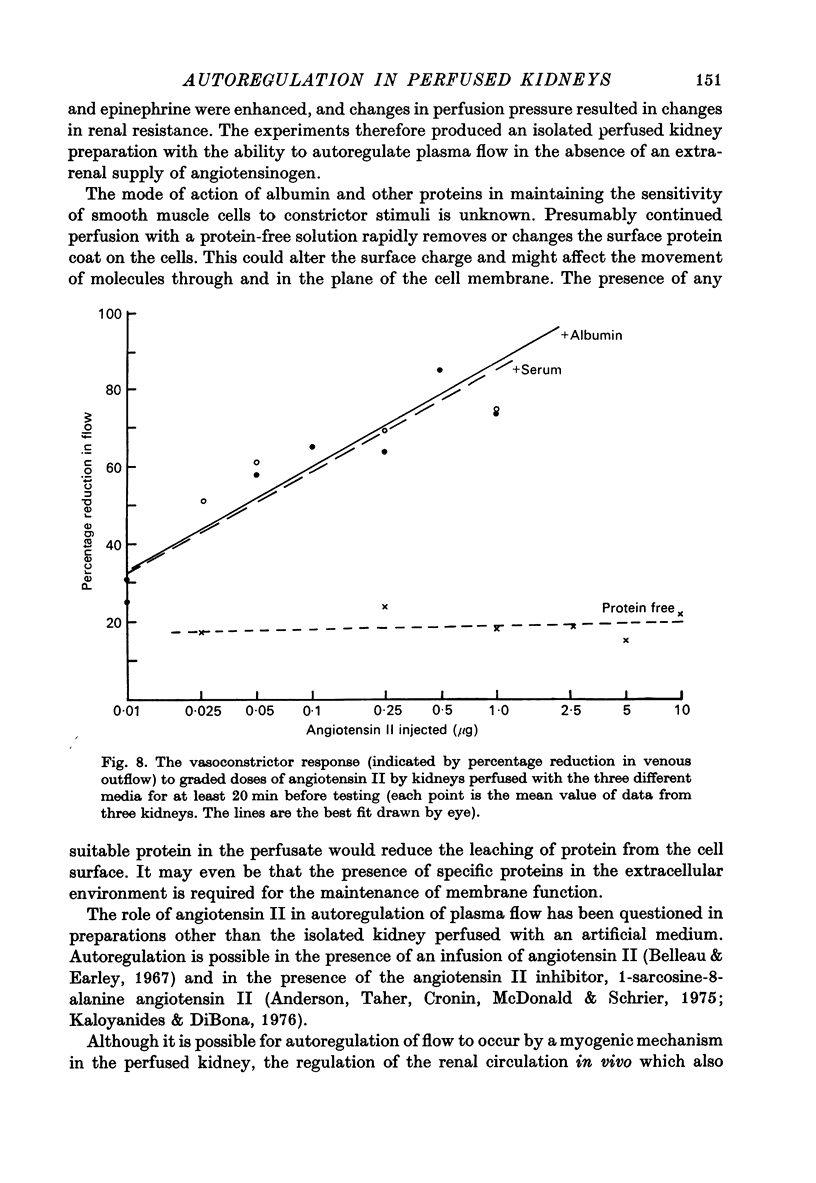
1. Autoregulation of renal plasma flow, by which flow remains constant despite changes in perfusion pressure, was studied in the isolated, perfused kidney of the rat. 2. Autoregulation did not occur in preparations perfused with a protein-free medium consisting of a balanced ionic solution resembling rat plasma in which 3% polyvinylpyrrolidone replaced the plasma proteins, changes in perfusion pressure over the normal autoregulatory range 100-150 mmHg produced a corresponding and linear change in venous outflow and no consistent change in renal vascular resistance. 3. Addition of human serum (5%, v/v) to the medium restored autoregulation; changes in perfusion pressure in the range 100-150 mmHg resulted in a stable plasma flow and a linear change in renal vascular resistance. The addition of bovine serum albumin (3 g/1.) to the protein-free medium restored autoregulation to a similar degree. 4. In kidneys perfused with the protein-free medium, the sensitivity of the renal vasculature to the vasoconstrictor drugs epinephrine and angiotensin II was only 1/40 the level seen in those kidneys perfused with media containing serum or albumin. 5. The experiments show that in the isolated, perfused kidney, autoregulation of plasma flow is not dependent on the presence of the globulin, angiotensinogen, in the perfusion medium; and suggest that failure of autoregulation in kidneys perfused with a protein-free medium could be attributed to the rapid decline in the sensitivity of the vascular smooth muscle to constrictor stimuli.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. J., Taher M. S., Cronin R. E., McDonald K. M., Schrier R. W. Effect of beta-adrenergic blockade and inhibitors of angiotensin II and prostaglandins on renal autoregulation. Am J Physiol. 1975 Sep;229(3):731–736. doi: 10.1152/ajplegacy.1975.229.3.731. [DOI] [PubMed] [Google Scholar]
- Arendshorst W. J., Finn W. F., Gottschalk C. W. Autoregulation of blood flow in the rat kidney. Am J Physiol. 1975 Jan;228(1):127–133. doi: 10.1152/ajplegacy.1975.228.1.127. [DOI] [PubMed] [Google Scholar]
- BULBRING E. Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol. 1955 Apr 28;128(1):200–221. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin-Legros M., Meyer P. Effects of angiotensin, catecholamines and cyclic AMP on calcium storage in aortic microsomes. Br J Pharmacol. 1973 Feb;47(2):377–385. doi: 10.1111/j.1476-5381.1973.tb08335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINSHAW L. B. MECHANISM OF RENAL AUTOREGULATION: ROLE OF TISSUE PRESSURE AND DESCRIPTION OF A MULTIFACTOR HYPOTHESIS. Circ Res. 1964 Aug;15:SUPPL–SUPPL:131. [PubMed] [Google Scholar]
- Johansson B., Bohr D. F. Rhythmic activity in smooth muscle from small subcutaneous arteries. Am J Physiol. 1966 Apr;210(4):801–806. doi: 10.1152/ajplegacy.1966.210.4.801. [DOI] [PubMed] [Google Scholar]
- Kaloyanides G. J., DiBona G. F. Effect of an angiotensin II antagonist on autoregulation in the isolated dog kidney. Am J Physiol. 1976 Apr;230(4):1078–1083. doi: 10.1152/ajplegacy.1976.230.4.1078. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., KINTER W. B. Hematocrit ratio of blood within mammalian kidney and its significance for renal hemodynamics. Am J Physiol. 1956 May;185(2):377–390. doi: 10.1152/ajplegacy.1956.185.2.377. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Pharmacology of excitation-contraction coupling in vascular smooth muscle and in avian slow muscle. Fed Proc. 1969 Sep-Oct;28(5):1634–1642. [PubMed] [Google Scholar]
- THURAU K. W. AUTOREGULATION OF RENAL BLOOD FLOW AND GLOMERULAR FILTRATION RATE, INCLUDING DATA ON TUBULAR AND PERITUBULAR CAPILLARY PRESSURES AND VESSEL WALL TENSION. Circ Res. 1964 Aug;15:SUPPL–SUPPL:141. [PubMed] [Google Scholar]
- THURAU K., KRAMER K. Weitere Untersuchungen zur myogenen Natur der Autoregulation des Nierenkreislaufes; Aufhebung der Autoregulation durch muskulotrope Substanzen und druckpassives Verhalten des Glomerulusfiltrates. Pflugers Arch. 1959;269(1):77–93. doi: 10.1007/BF00362973. [DOI] [PubMed] [Google Scholar]
- WAUGH W. H. CIRCULATORY AUTOREGULATION IN THE FULLY ISOLATED KIDNEY AND IN THE HUMORALLY SUPPORTED, ISOLATED KIDNEY. Circ Res. 1964 Aug;15:SUPPL–SUPPL:169. [PubMed] [Google Scholar]
- WEISS C., PASSOW H., ROTHSTEIN A. Autoregulation of flow in isolated rat kidney in the absence of red cells. Am J Physiol. 1959 May;196(5):1115–1118. doi: 10.1152/ajplegacy.1959.196.5.1115. [DOI] [PubMed] [Google Scholar]
- WURZEL M., BACON R. C., KALT R. B., ZWEIFACH B. W. VASOACTIVE PROPERTIES OF PLASMA PROTEIN FRACTIONS. Am J Physiol. 1964 Apr;206:923–925. doi: 10.1152/ajplegacy.1964.206.4.923. [DOI] [PubMed] [Google Scholar]


