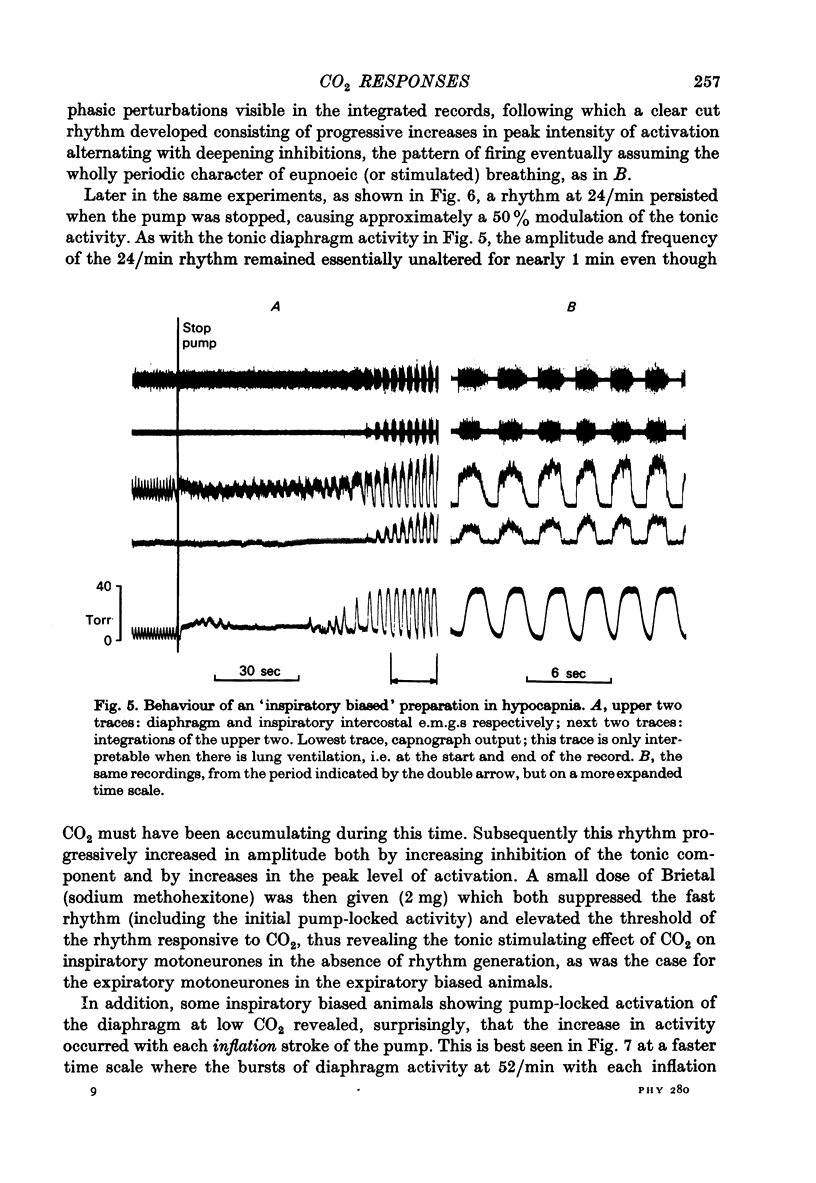
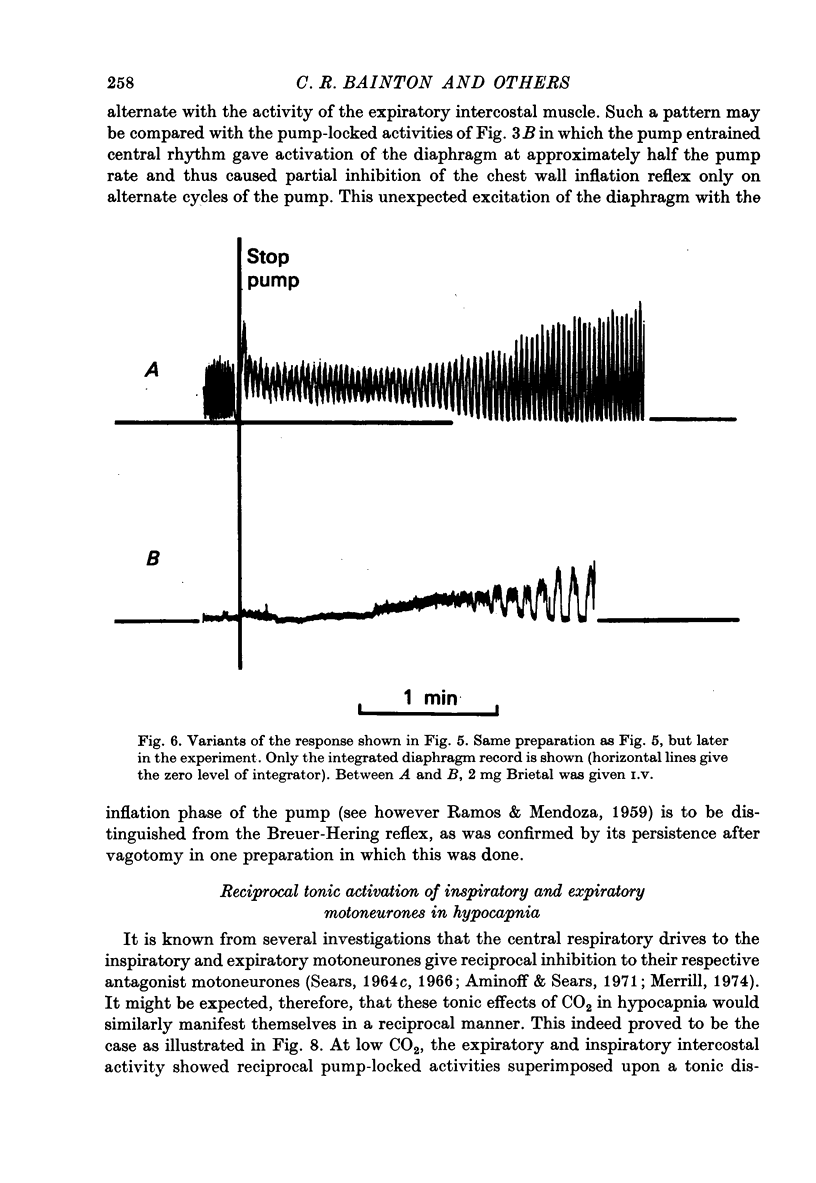
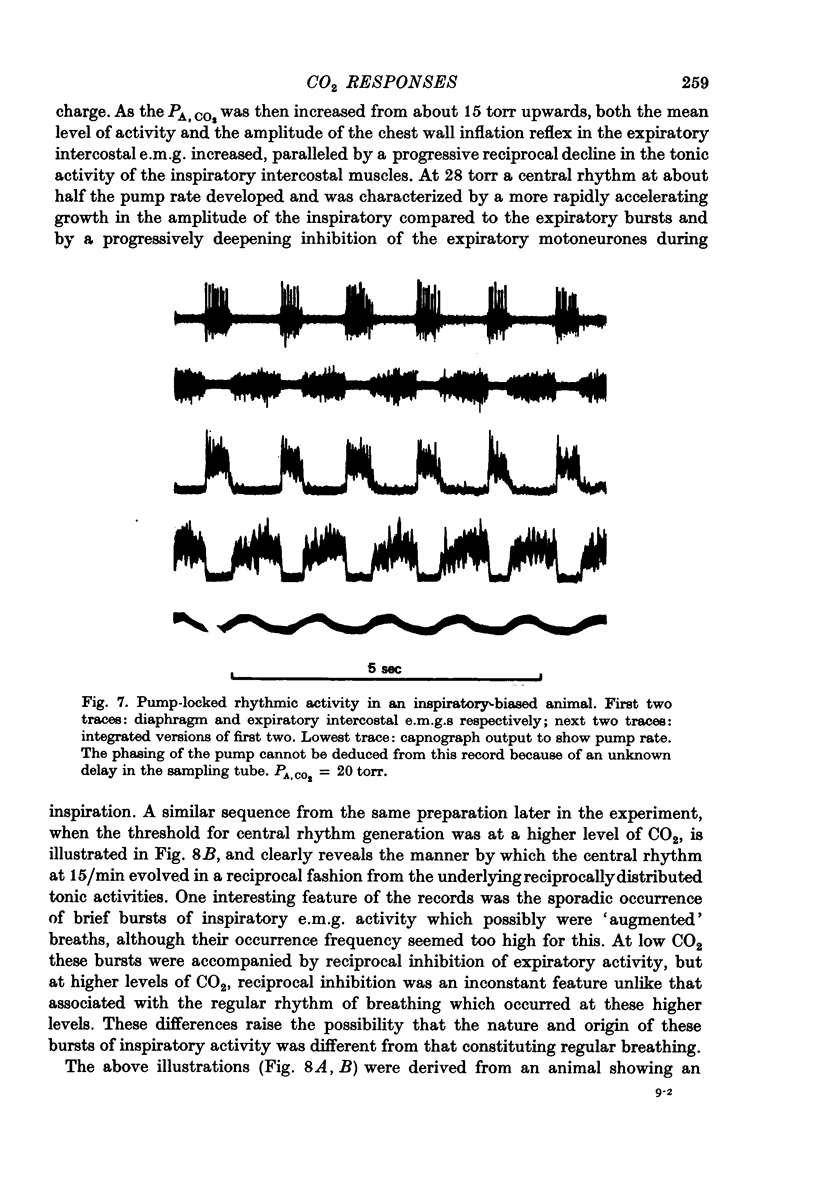
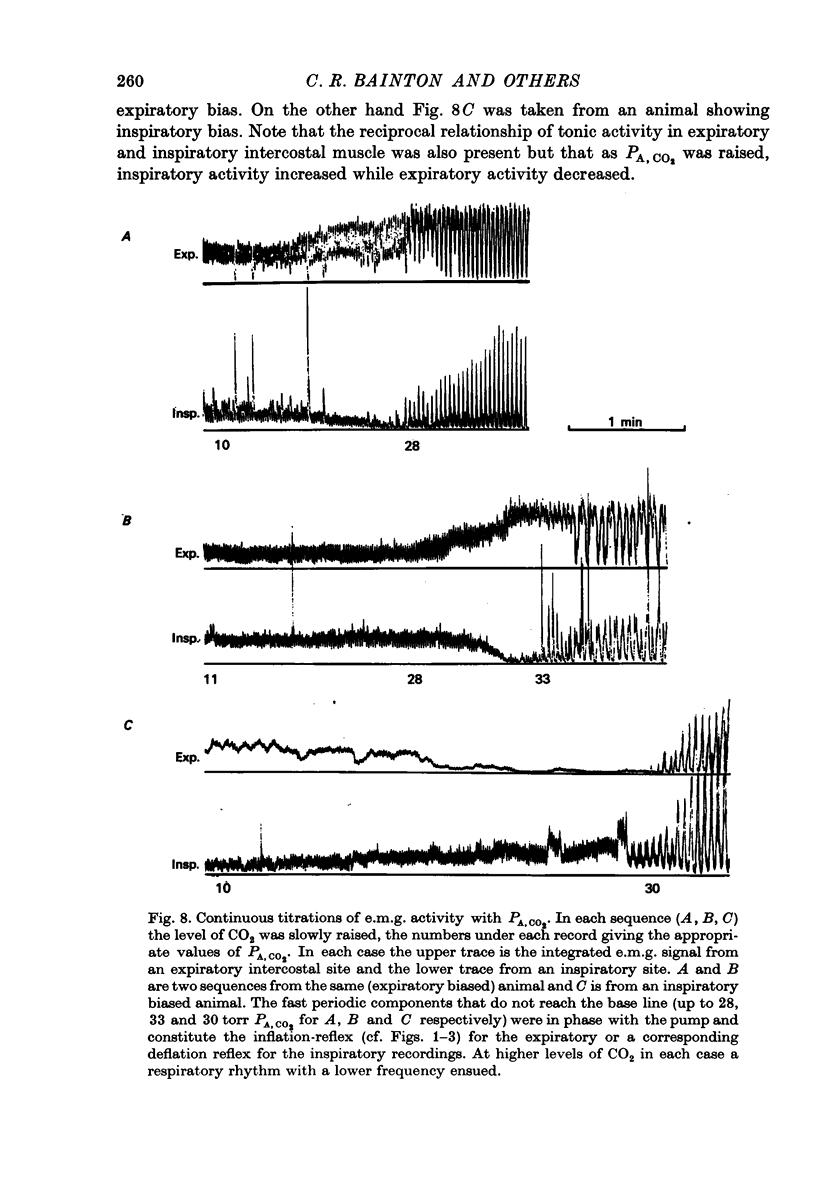
Abstract
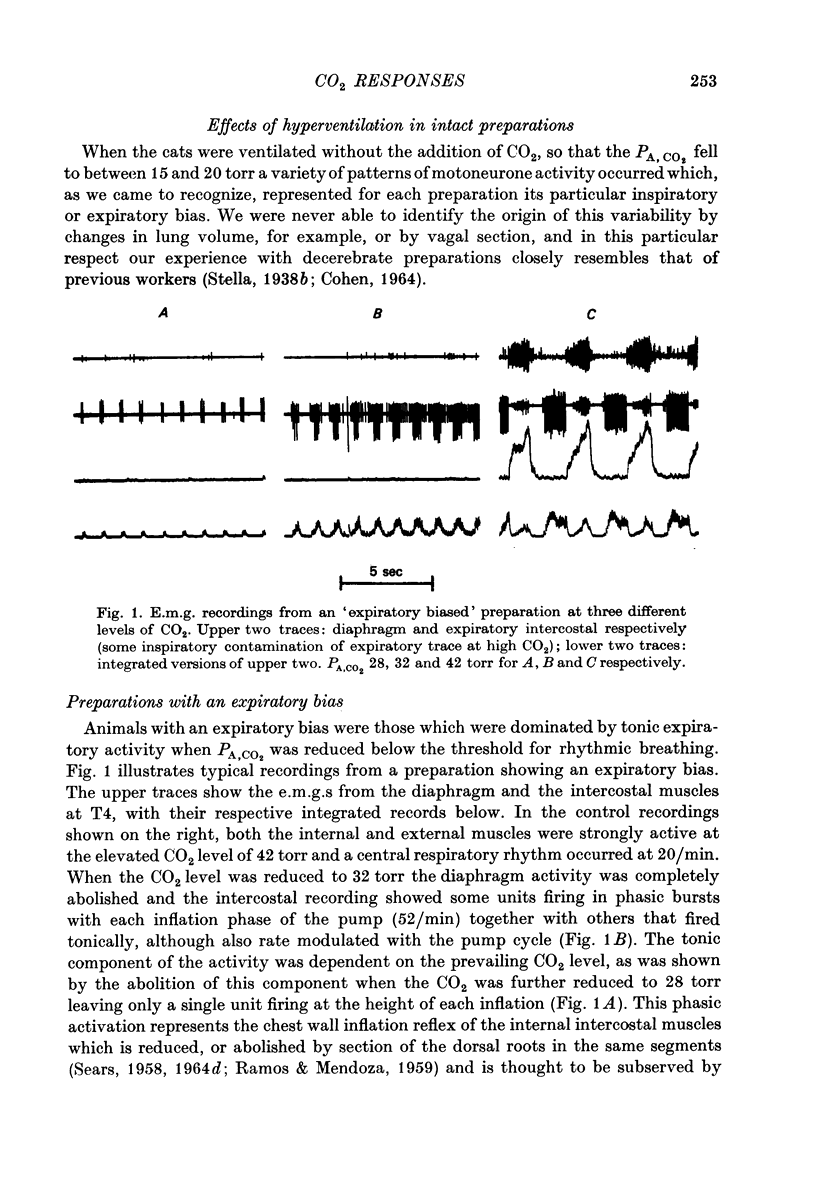
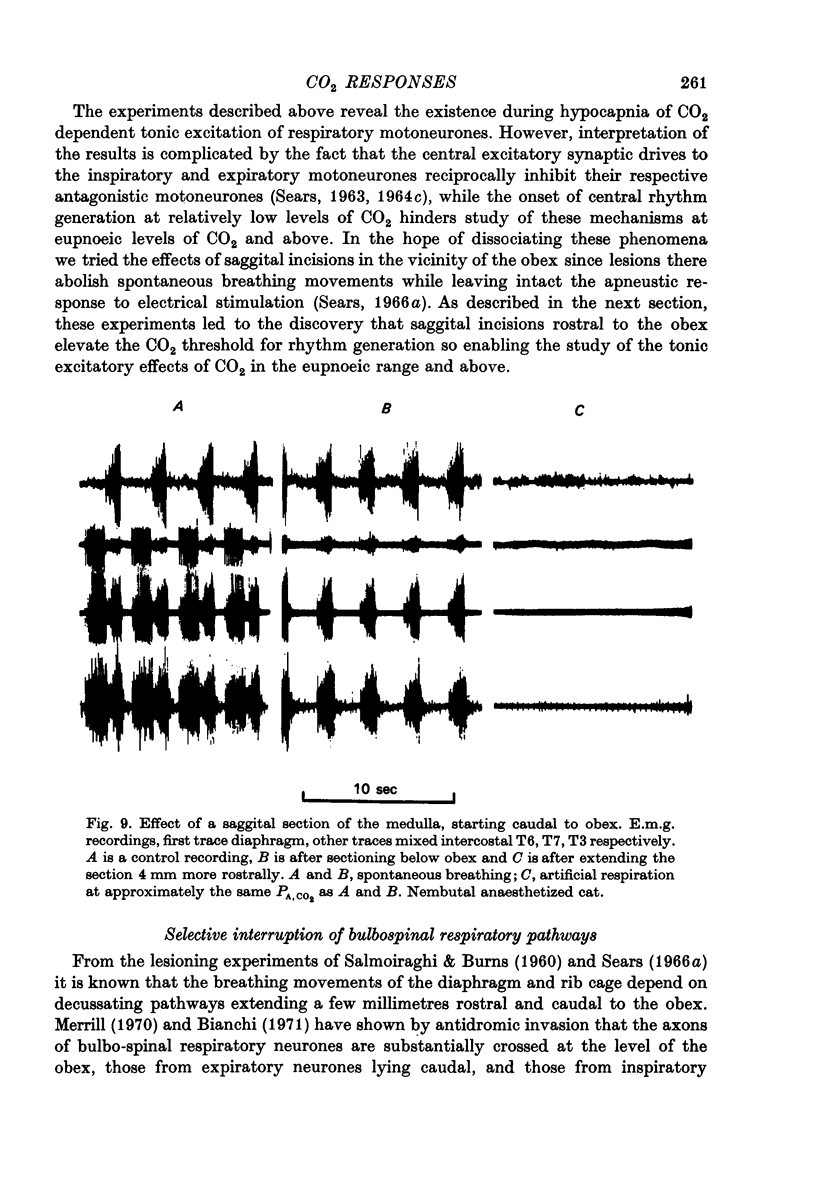
1. Electromyography was used to measure the response of the diaphragm and intercostal muscles to CO2 in artificially ventilated decerebrate cats. 2. Hypocapnia produced tonic activity in either inspiratory or expiratory muscles or both, according to the preparation. 3. A graded effect of CO2 on both rhythmic and tonic activity was observed and for the latter this could be seen at as low as 10 torr PA,CO2. 4. In one human subject tonic firing of expiratory motoneurones was also induced by hypocapnia and this activity showed a graded increase with increasing (CO2. 5. A saggital incision of the medulla aimed at interrupting inspiratory bulbospinal axons abolished activity in inspiratory muscles and at eupnoeic levels of CO2 converted the activity of expiratory muscles from a periodic to a topic firing pattern. 6. Following such lesions the threshold for rhythmic excitation of expiratory muscles was elevated and this revealed that the graded effect of CO2 on tonic expiratory activity extends to as high as 60 torr. 7. The tonic activation of respiratory muscles in response to CO2 ceased after cervical cord transection or when the saggital incision in the medulla was extended caudally to the first cervical segment. 8. It is concluded that the CO2 dependent activation of spinal respiratory motoneurones is conveyed by bulbospinal axons which decussate in the vicinity of the obex and that this activation can be rhythmic or tonic. 9. It is suggested that the rhythmic excitation of expiratory muscles derives from a periodic inhibition of expiratory bulbospinal neurones which are subjected to a tonic CO2 dependent excitation which is continuously variable over the physiological range.
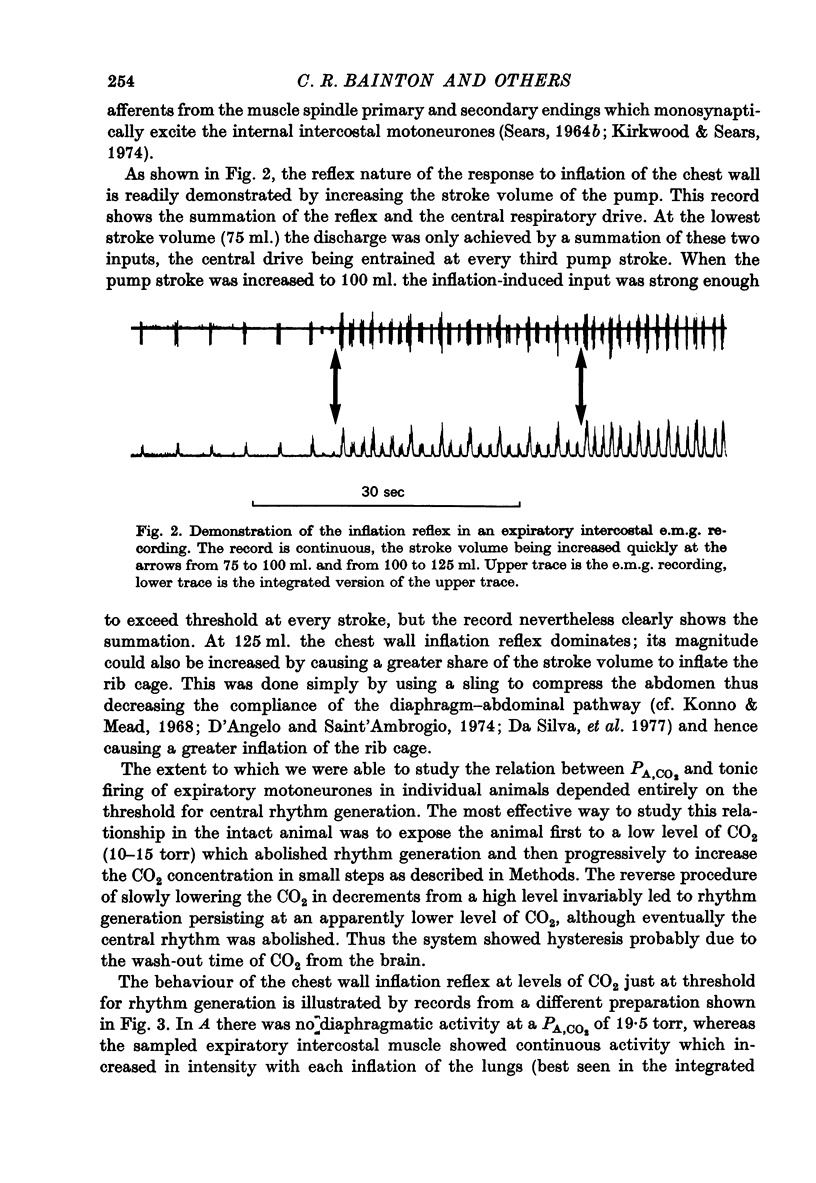
Full text
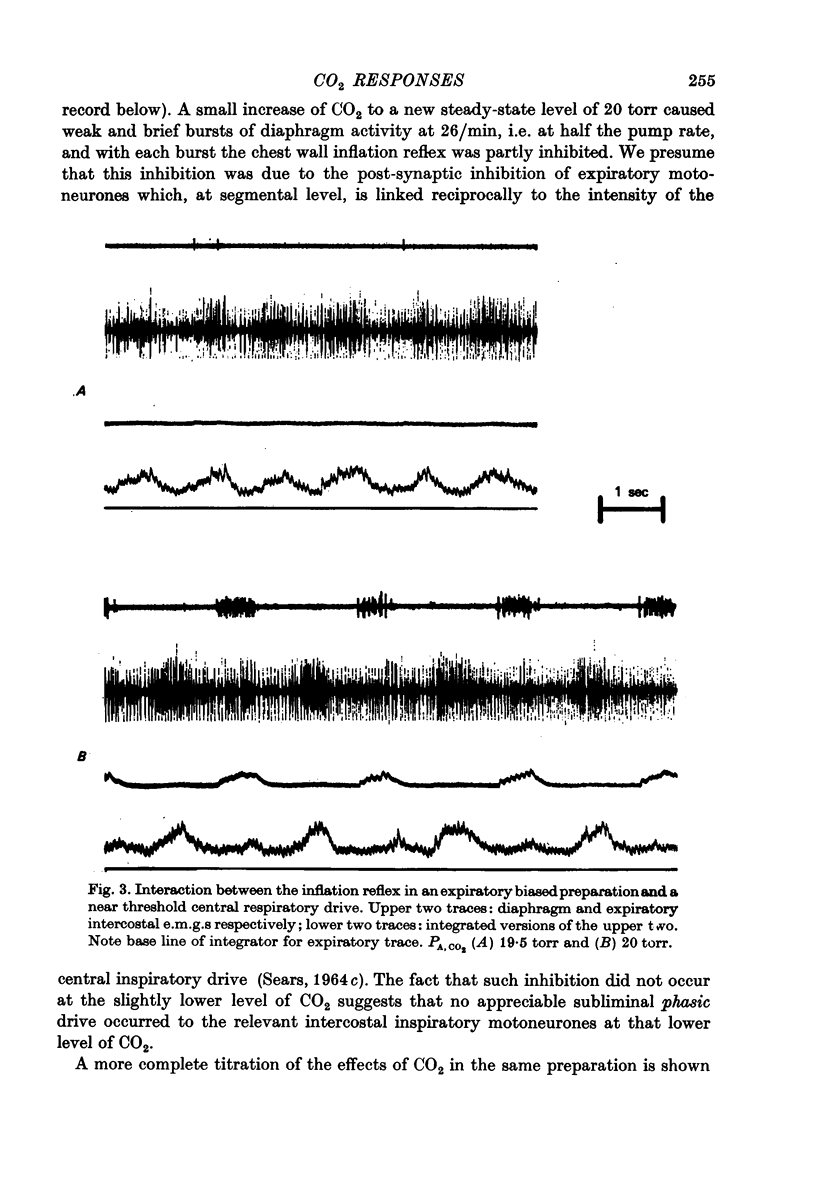
PDF



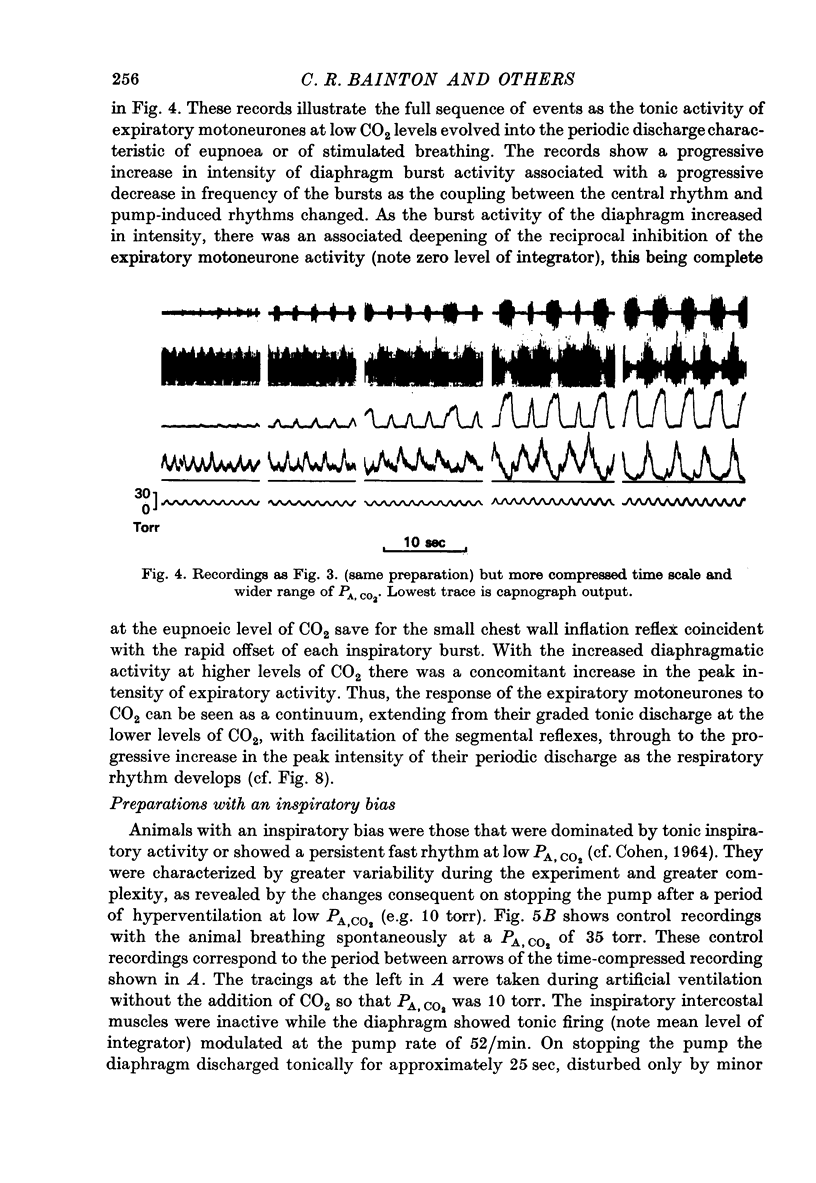
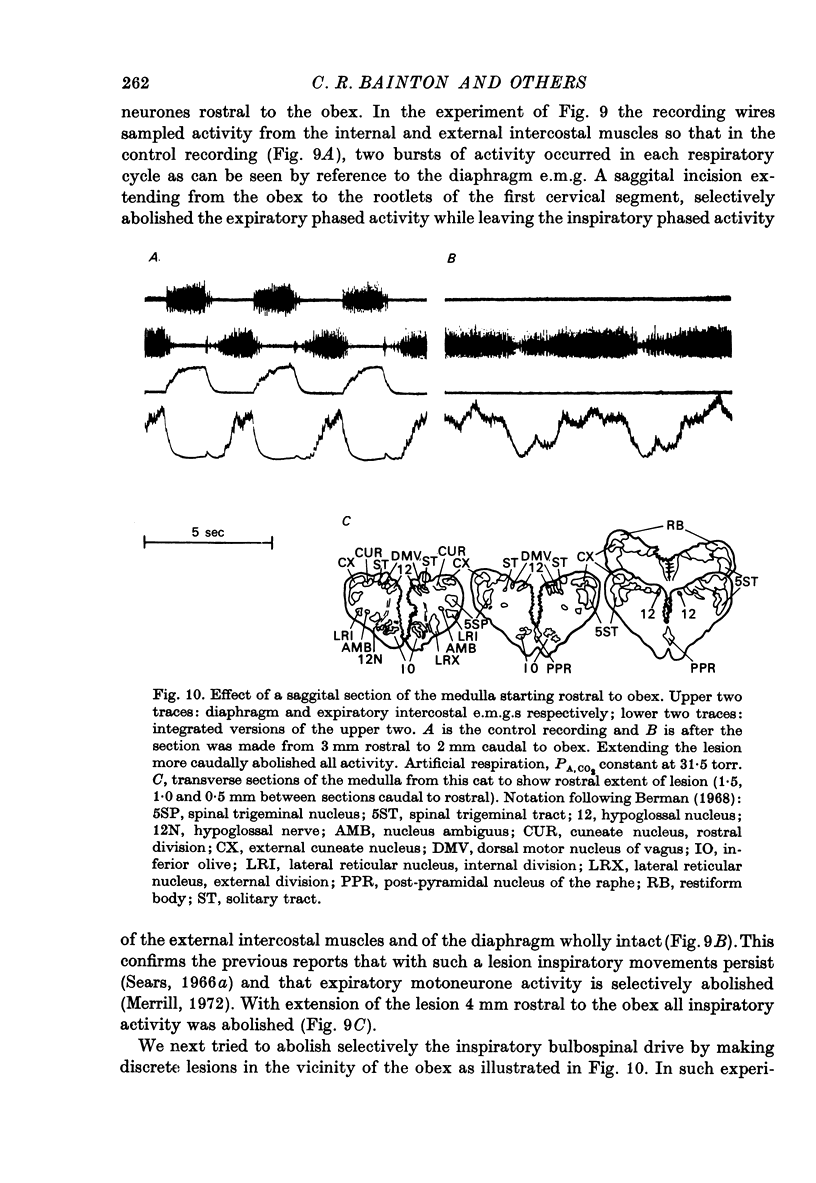
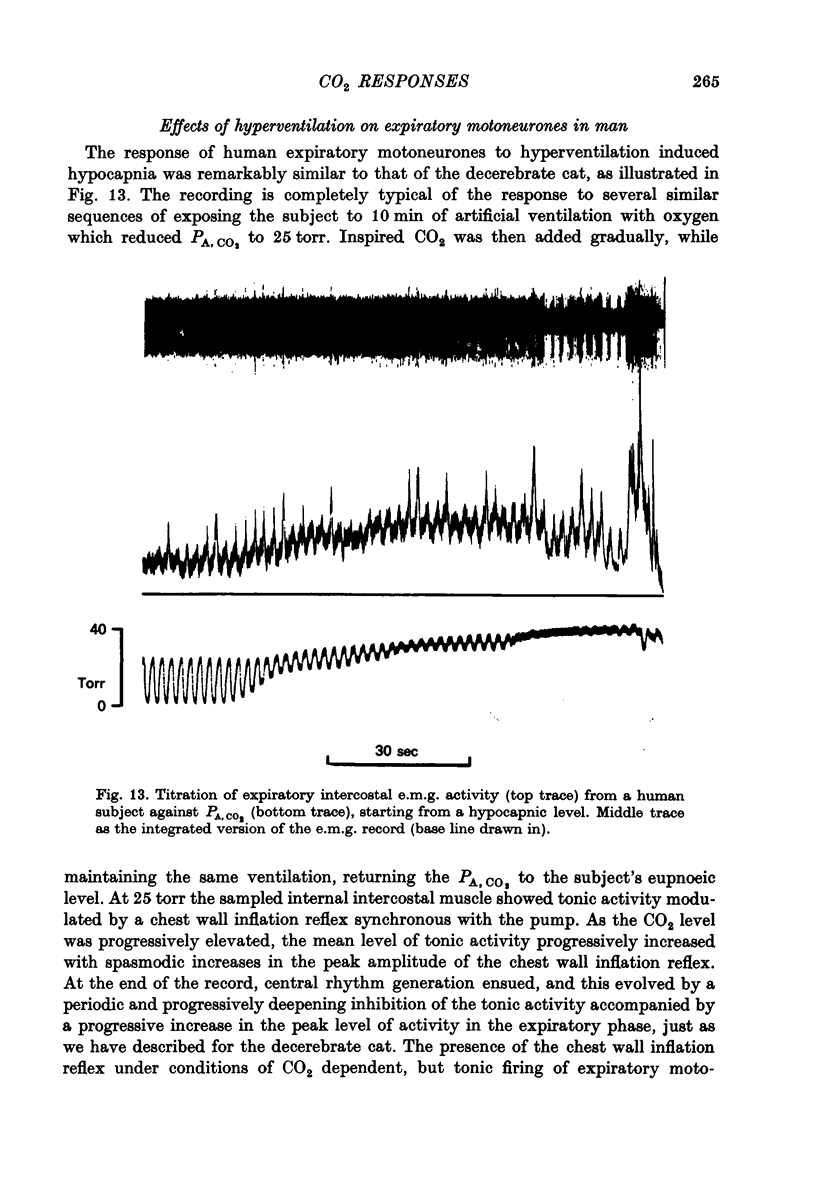

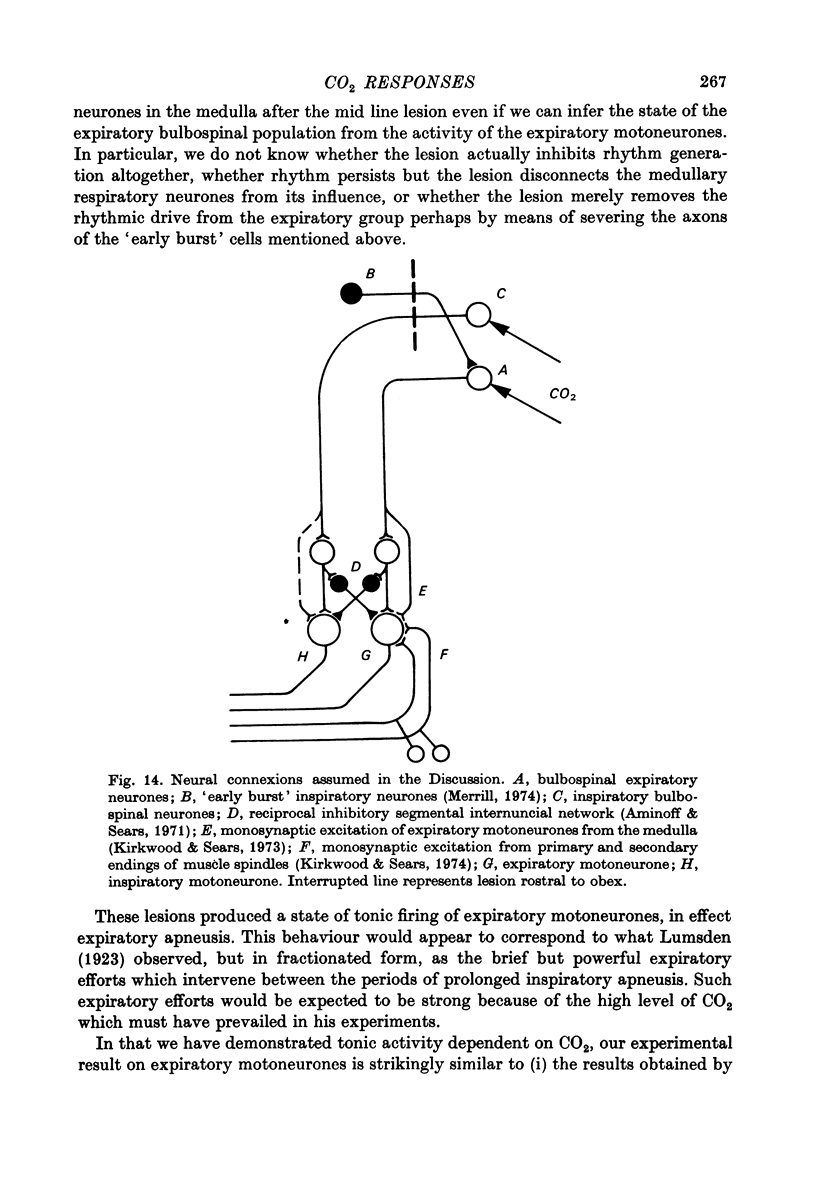





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
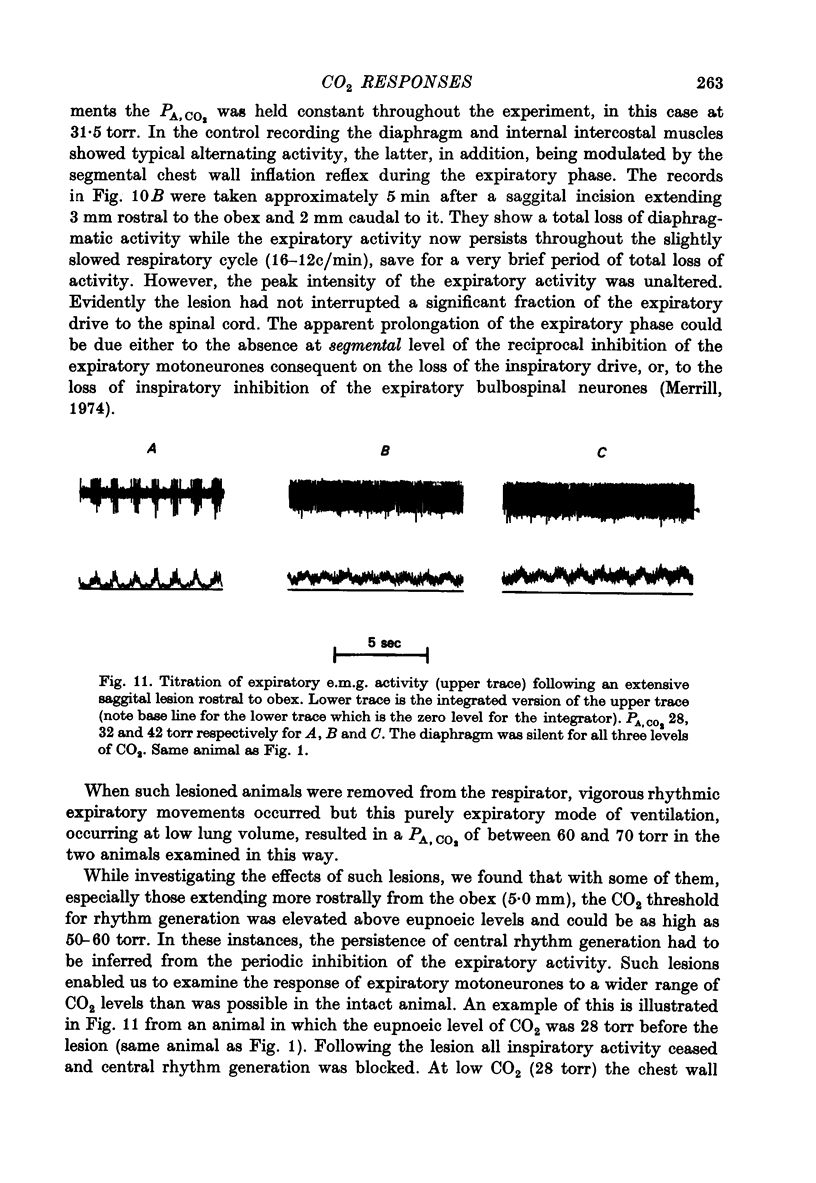
- ACHARD O., BUCHER V. M. Courants d'action bulbaires à rythme respiratoire. Helv Physiol Pharmacol Acta. 1954;12(4):265–283. [PubMed] [Google Scholar]
- Aminoff M. J., Sears T. A. Spinal integration of segmental, cortical and breathing inputs to thoracic respiratory motoneurones. J Physiol. 1971 Jun;215(2):557–575. doi: 10.1113/jphysiol.1971.sp009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
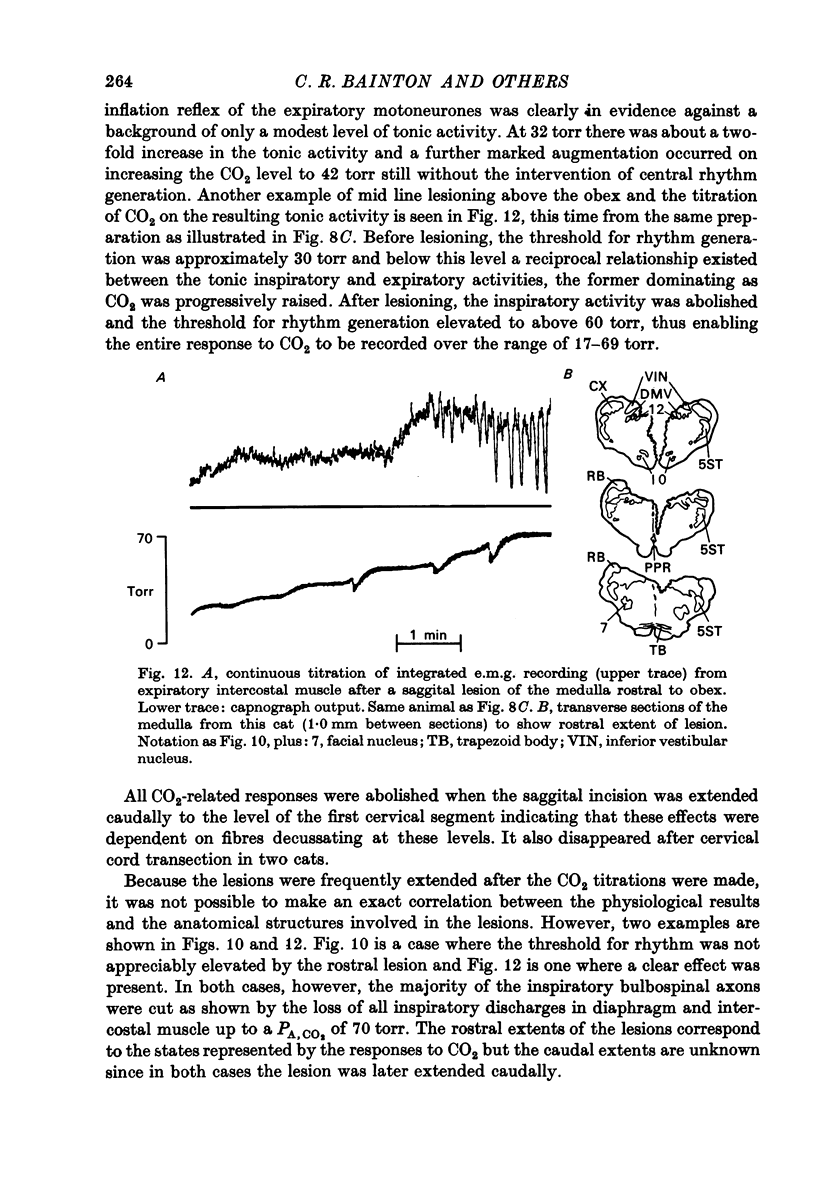
- Bainton C. R., Kirkwood P. A., Sears T. A. Proceedings: The response of expiratory motoneurones to CO2 in the absence of rhythmic breathing. J Physiol. 1974 Sep;241(2):122P–123P. [PubMed] [Google Scholar]
- Bainton C. R., Mitchell R. A. Posthyperventilation apnea in awake man. J Appl Physiol. 1966 Mar;21(2):411–415. doi: 10.1152/jappl.1966.21.2.411. [DOI] [PubMed] [Google Scholar]
- Batsel H. L. Activity of bulbar respiratory neurons during passive hyperventilation. Exp Neurol. 1967 Nov;19(3):357–374. doi: 10.1016/0014-4886(67)90032-5. [DOI] [PubMed] [Google Scholar]
- Berger A. J. Dorsal respiratory group neurons in the medulla of cat: spinal projections, responses to lung inflation and superior laryngeal nerve stimulation. Brain Res. 1977 Oct 28;135(2):231–254. doi: 10.1016/0006-8993(77)91028-9. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J. Carotid body: structure and function. Physiol Rev. 1971 Jul;51(3):437–495. doi: 10.1152/physrev.1971.51.3.437. [DOI] [PubMed] [Google Scholar]
- COHEN M. I. RESPIRATORY PERIODICITY IN THE PARALYZED, VAGOTOMIZED CAT: HYPOCAPNIC POLYPNEA. Am J Physiol. 1964 Apr;206:845–854. doi: 10.1152/ajplegacy.1964.206.4.845. [DOI] [PubMed] [Google Scholar]
- Cohen M. I. Discharge patterns of brain-stem respiratory neurons in relation to carbon dioxide tension. J Neurophysiol. 1968 Mar;31(2):142–165. doi: 10.1152/jn.1968.31.2.142. [DOI] [PubMed] [Google Scholar]
- Da Silva K. M., Sayers B. M., Sears T. A., Stagg D. T. The changes in configuration of the rib cage and abdomen during breathing in the anaesthetized cat. J Physiol. 1977 Apr;266(2):499–521. doi: 10.1113/jphysiol.1977.sp011779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. O., Edwards M. W., Jr Medullary relay neurons in the carotid-body chemoreceptor pathway of cats. Respir Physiol. 1975 Jun;24(1):69–79. doi: 10.1016/0034-5687(75)90122-x. [DOI] [PubMed] [Google Scholar]
- Davis J. N., Sears T. A. The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol. 1970 Aug;209(3):711–738. doi: 10.1113/jphysiol.1970.sp009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decima E. E., von Euler C. Intercostal and cerebellar influences on efferent phrenic activity in the decerebrate cat. Acta Physiol Scand. 1969 May-Jun;76(1):148–158. doi: 10.1111/j.1748-1716.1969.tb04459.x. [DOI] [PubMed] [Google Scholar]
- Decima E. E., von Euler C., Thoden U. Intercostal-to-phrenic reflexes in the spinal cat. Acta Physiol Scand. 1969 Apr;75(4):568–579. [PubMed] [Google Scholar]
- Duron B. Postural and ventilatory functions of intercostal muscles. Acta Neurobiol Exp (Wars) 1973;33(1):355–380. [PubMed] [Google Scholar]
- EKLUND G., VON EULER, RUTKOWSKI S. SPONTANEOUS AND REFLEX ACTIVITY OF INTERCOSTAL GAMMA MOTONEURONES. J Physiol. 1964 May;171:139–163. doi: 10.1113/jphysiol.1964.sp007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L. Expiratory effects of brief carotid sinus nerve and carotid body stimulations. Respir Physiol. 1976 May;26(3):395–410. doi: 10.1016/0034-5687(76)90009-8. [DOI] [PubMed] [Google Scholar]
- Fallert M., Böhmer G., Dinse H. R. Patterns of bulbar respiratory neurons during and after artifical hyperventilation. Respir Physiol. 1977 Apr;29(2):143–149. doi: 10.1016/0034-5687(77)90087-1. [DOI] [PubMed] [Google Scholar]
- GARCIA RAMOS J., LOPEZ MENDOZA E. On the integration of respiratory movements. II. The integration at spinal level. Acta Physiol Lat Am. 1959;9:257–266. [PubMed] [Google Scholar]
- HUKUHARA T., NAKAYAMA S., OKADA H. Action potentials in the normal respiratory centers and its centrifugal pathways in the medulla oblongata and spinal cord. Jpn J Physiol. 1954 Jun 30;4(2):145–153. doi: 10.2170/jjphysiol.4.145. [DOI] [PubMed] [Google Scholar]
- Head H. On the Reglation of Respiration: PART I. Experimental. J Physiol. 1889 Feb;10(1-2):1–152.53. doi: 10.1113/jphysiol.1889.sp000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from secondary endings of muscle spindles. Nature. 1974 Nov 15;252(5480):243–244. doi: 10.1038/252243a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Proceedings: Monosynaptic excitation of thoracic expiratory motoneurones from lateral respiratory neurones in the medulla of the cat. J Physiol. 1973 Oct;234(2):87P–89P. [PubMed] [Google Scholar]
- Konno K., Mead J. Static volume-pressure characteristics of the rib cage and abdomen. J Appl Physiol. 1968 Apr;24(4):544–548. doi: 10.1152/jappl.1968.24.4.544. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. T., Boyarsky L. L. Neurophysiological investigations of medullary chemosensitive areas of respiration. Respir Physiol. 1972 Dec;16(3):362–376. doi: 10.1016/0034-5687(72)90065-5. [DOI] [PubMed] [Google Scholar]
- Loeschcke H. H., De Lattre J., Schläfke M. E., Trouth C. O. Effects on respiration and circulation of electrically stimulating the ventral surface of the medulla oblongata. Respir Physiol. 1970 Sep;10(2):184–197. doi: 10.1016/0034-5687(70)90082-4. [DOI] [PubMed] [Google Scholar]
- Lumsden T. The regulation of respiration: Part I. J Physiol. 1923 Oct 22;58(1):81–91. doi: 10.1113/jphysiol.1923.sp002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSION J., MEULDERS M., COLLE J. [Postural function of the respiratory muscles]. Arch Int Physiol Biochim. 1960 Mar;68:314–326. doi: 10.3109/13813456009083552. [DOI] [PubMed] [Google Scholar]
- Merrill E. G. The lateral respiratory neurones of the medulla: their associations with nucleus ambiguus, nucleus retroambigualis, the spinal accessory nucleus and the spinal cord. Brain Res. 1970 Nov 11;24(1):11–28. doi: 10.1016/0006-8993(70)90271-4. [DOI] [PubMed] [Google Scholar]
- Merrill E. G. Thoracic motor drives from medullary expiratory neurones in cats. J Physiol. 1972 Apr;222(2):154P–155P. [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A. Synchronized high frequency synaptic potentials in medullary respiratory neurons. Brain Res. 1974 Jul 26;75(2):350–355. doi: 10.1016/0006-8993(74)90760-4. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., FENCL V., HEISEY S. R., HELD D. ROLE OF CEREBRAL FLUIDS IN CONTROL OF RESPIRATION AS STUDIED IN UNANESTHETIZED GOATS. Am J Physiol. 1965 Mar;208:436–450. doi: 10.1152/ajplegacy.1965.208.3.436. [DOI] [PubMed] [Google Scholar]
- Remmers J. E., Tsiaras W. G. Effect of lateral cervical cord lesions on the respiratory rhythm of anaesthetized, decerebrate cats after vagotomy. J Physiol. 1973 Aug;233(1):63–74. doi: 10.1113/jphysiol.1973.sp010297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS T. A. Activity of fusimotor fibres innervating muscle spindles in the intercostal muscles of the cat. Nature. 1963 Mar 9;197:1013–1014. doi: 10.1038/1971013a0. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. EFFERENT DISCHARGES IN ALPHA AND FUSIMOTOR FIBRES OF INTERCOSTAL NERVES OF THE CAT. J Physiol. 1964 Nov;174:295–315. doi: 10.1113/jphysiol.1964.sp007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS T. A. INVESTIGATIONS ON RESPIRATORY MOTONEURONES OF THE THORACIC SPINAL CORD. Prog Brain Res. 1964;12:259–273. doi: 10.1016/s0079-6123(08)60627-5. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. SOME PROPERTIES AND REFLEX CONNEXIONS OF RESPIRATORY MOTONEURONES OF THE CAT'S THORACIC SPINAL CORD. J Physiol. 1964 Dec;175:386–403. doi: 10.1113/jphysiol.1964.sp007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS T. A. THE SLOW POTENTIALS OF THORACIC RESPIRATORY MOTONEURONES AND THEIR RELATION TO BREATHING. J Physiol. 1964 Dec;175:404–424. doi: 10.1113/jphysiol.1964.sp007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaefke M. E., See W. R., Loeschcke H. H. Ventilatory response to alterations of H+ ion concentration in small areas of the ventral medullary surface. Respir Physiol. 1970 Sep;10(2):198–212. doi: 10.1016/0034-5687(70)90083-6. [DOI] [PubMed] [Google Scholar]
- Sears T. A., Kirkwood P. A., Bainton C. R. Proceedings: Medullary-spinal neural connectivity and breathing. Bull Physiopathol Respir (Nancy) 1975 Mar-Apr;11(2):82P–83P. [PubMed] [Google Scholar]
- Sears T. A. The respiratory motoneuron and apneusis. Fed Proc. 1977 Sep;36(10):2412–2420. [PubMed] [Google Scholar]
- Stella G. On the mechanism of production, and the physiological significance of "apneusis". J Physiol. 1938 Jun 14;93(1):10–23. doi: 10.1113/jphysiol.1938.sp003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella G. The dependence of the activity of the "apneustic centre" on the carbon dioxide of the arterial blood. J Physiol. 1938 Aug 15;93(3):263–275. doi: 10.1113/jphysiol.1938.sp003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. The contribution of the intercostal muscles to the effort of respiration in man. J Physiol. 1960 May;151:390–402. doi: 10.1113/jphysiol.1960.sp006446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert J. F., Bertrand F., Denavit-Saubié M., Hugelin A. Three dimensional representation of bulbo-pontine respiratory networks architecture from unit density maps. Brain Res. 1976 Sep 17;114(2):227–244. doi: 10.1016/0006-8993(76)90668-5. [DOI] [PubMed] [Google Scholar]
- WYSS O. A. The part played by the lungs in the reflex control of breathing. Helv Physiol Pharmacol Acta Suppl 1. 1954 Dec;10:26–35. [PubMed] [Google Scholar]
- von BAUMGARTEN, BALTHASAR K., KOEPCHEN H. P. [On a substrate respiration-rhythmic stimulus formation in the cat rhombencephalon]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:504–528. [PubMed] [Google Scholar]
- von Euler C., Marttila I., Remmers J. E., Trippenbach T. Effects of lesions in the parabrachial nucleus on the mechanisms for central and reflex termination of inspiration in the cat. Acta Physiol Scand. 1976 Mar;96(3):324–337. doi: 10.1111/j.1748-1716.1976.tb10203.x. [DOI] [PubMed] [Google Scholar]


