Abstract
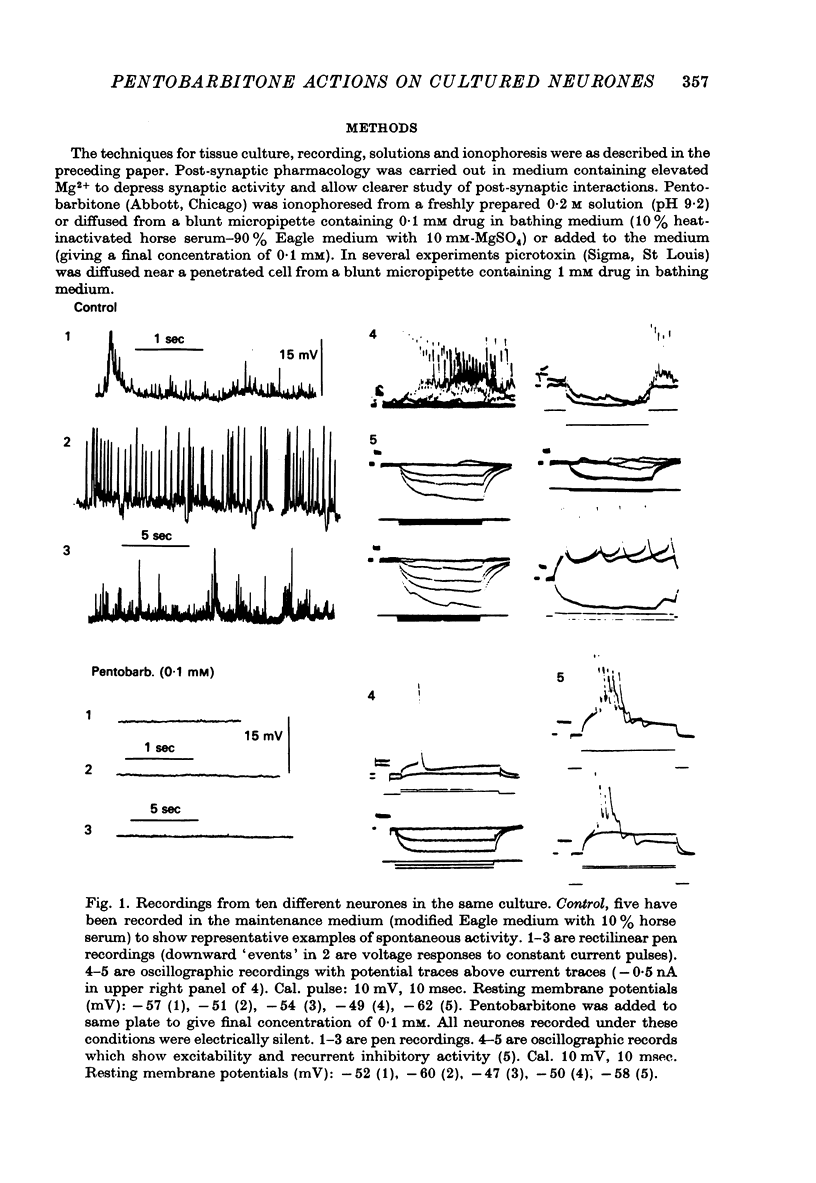
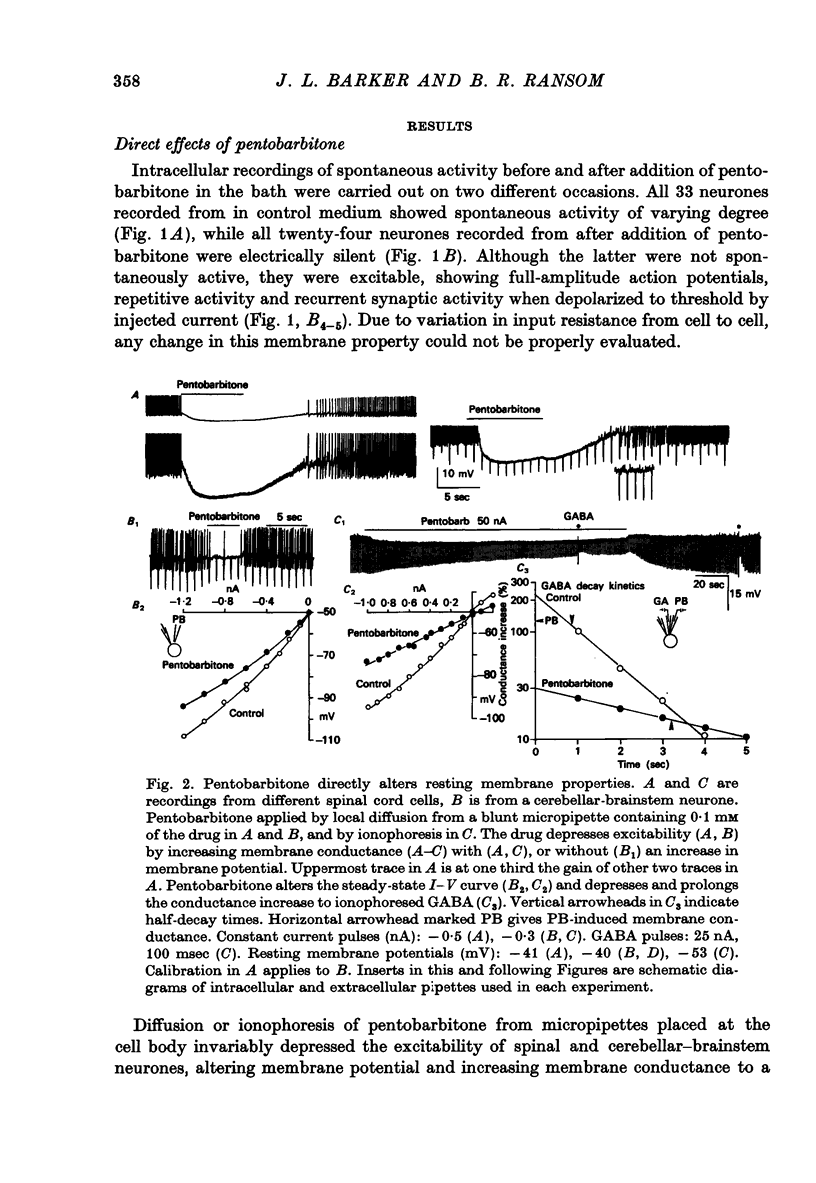
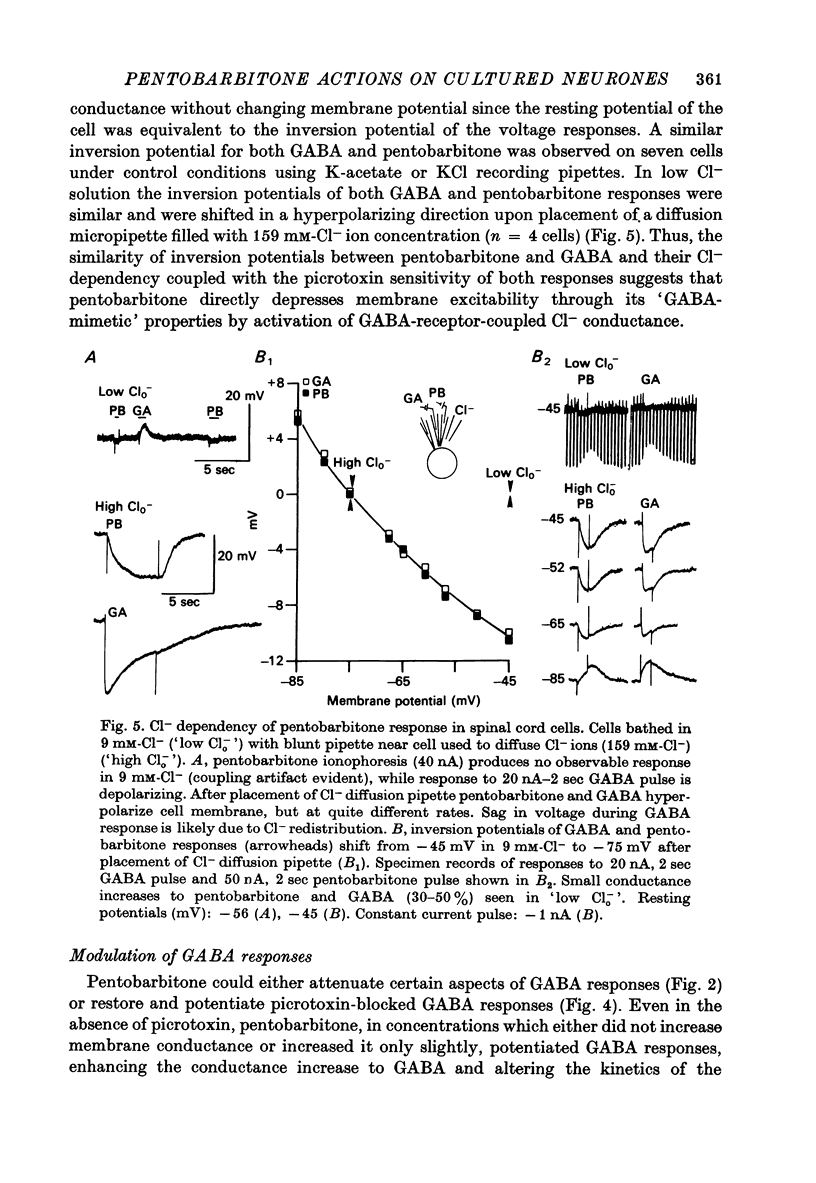
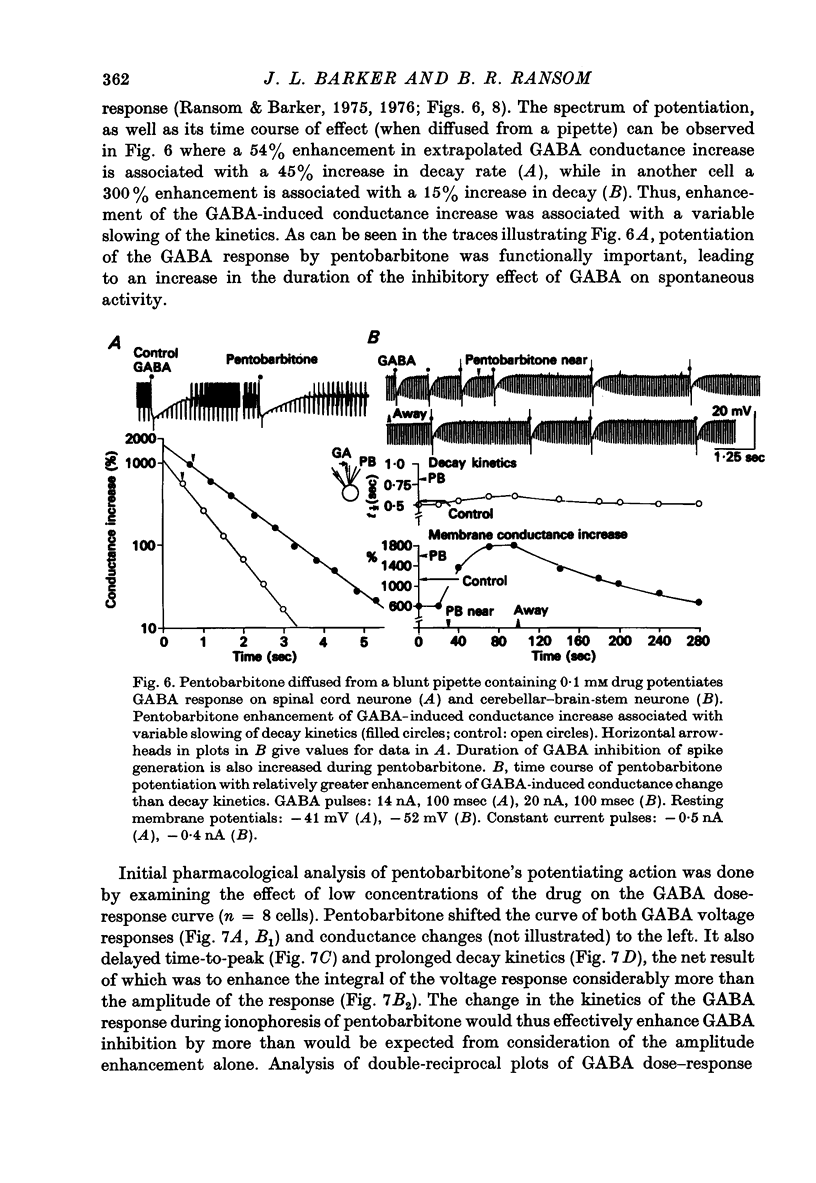
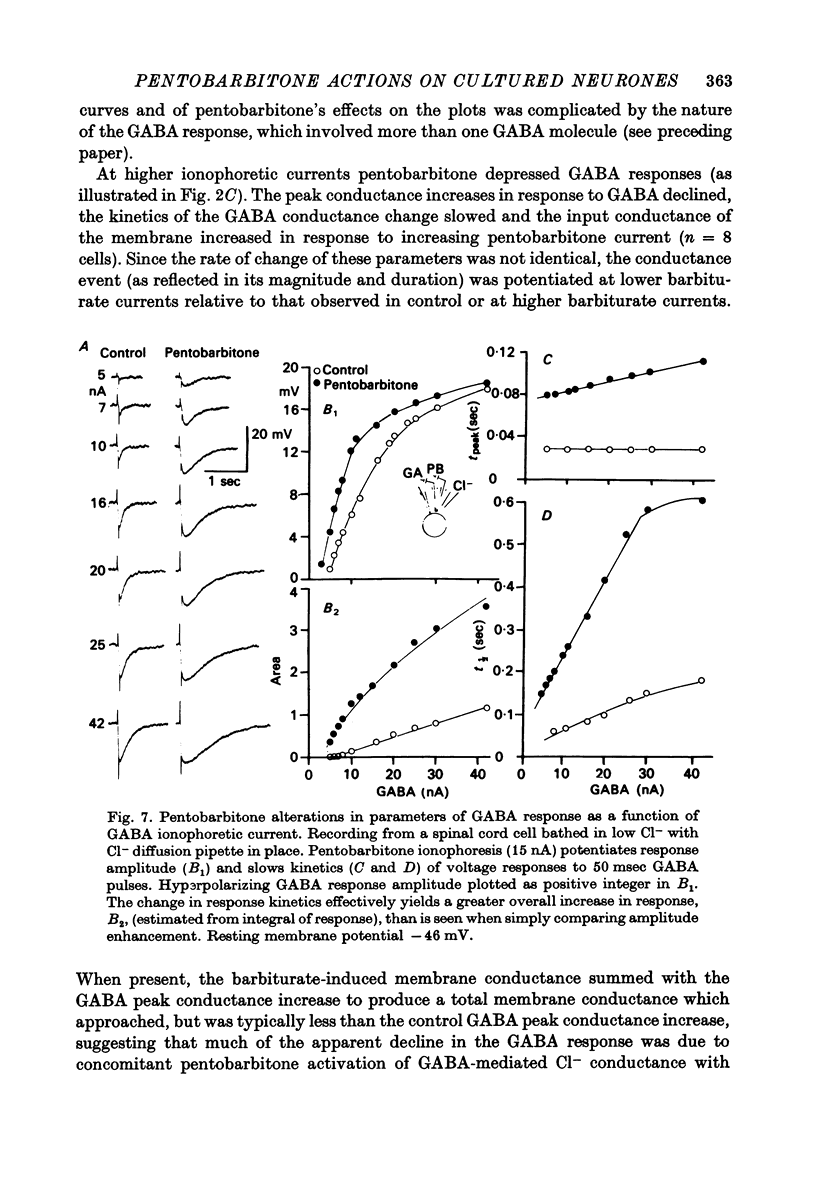
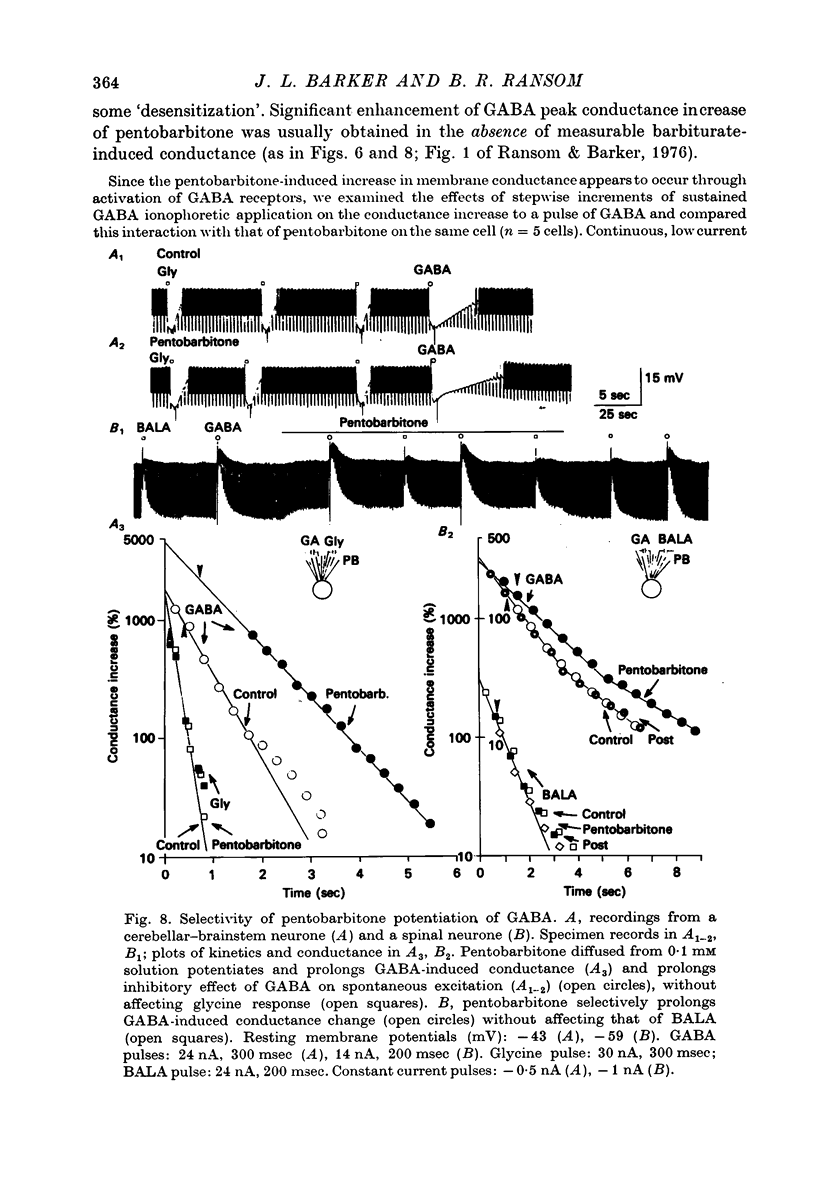
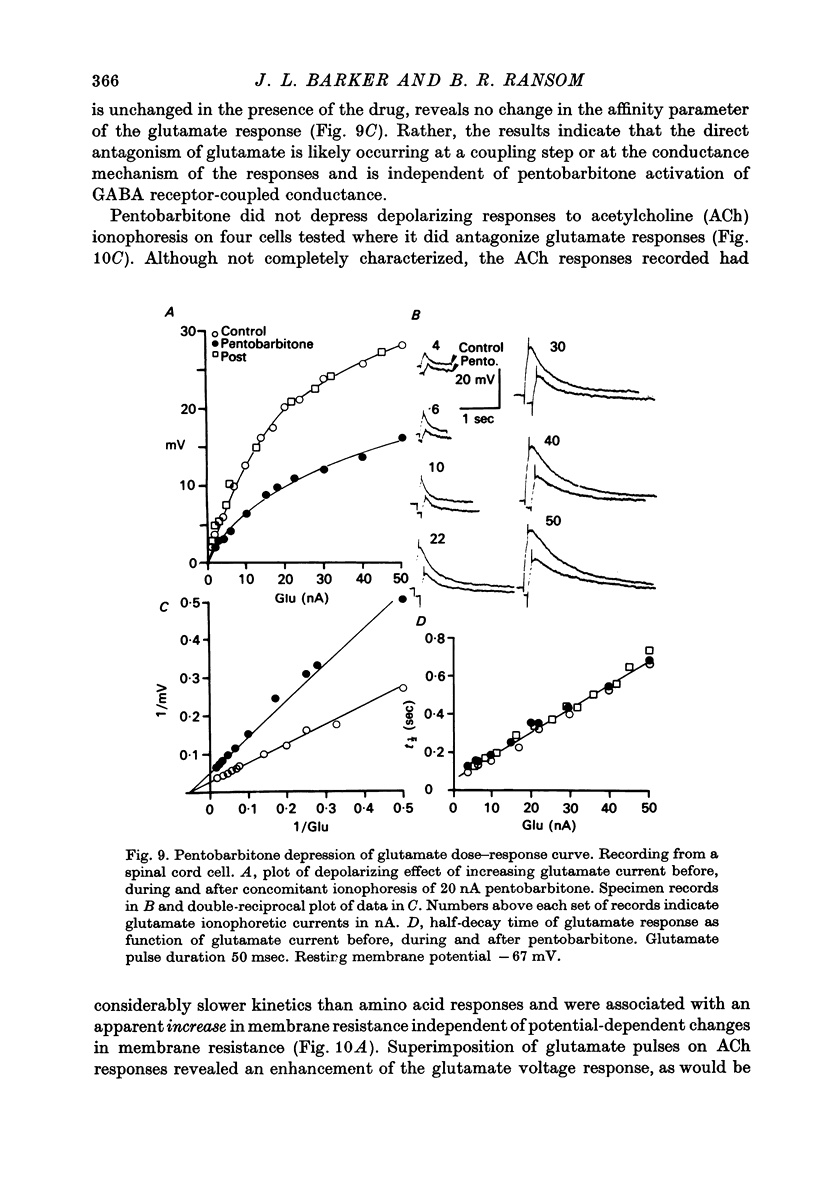
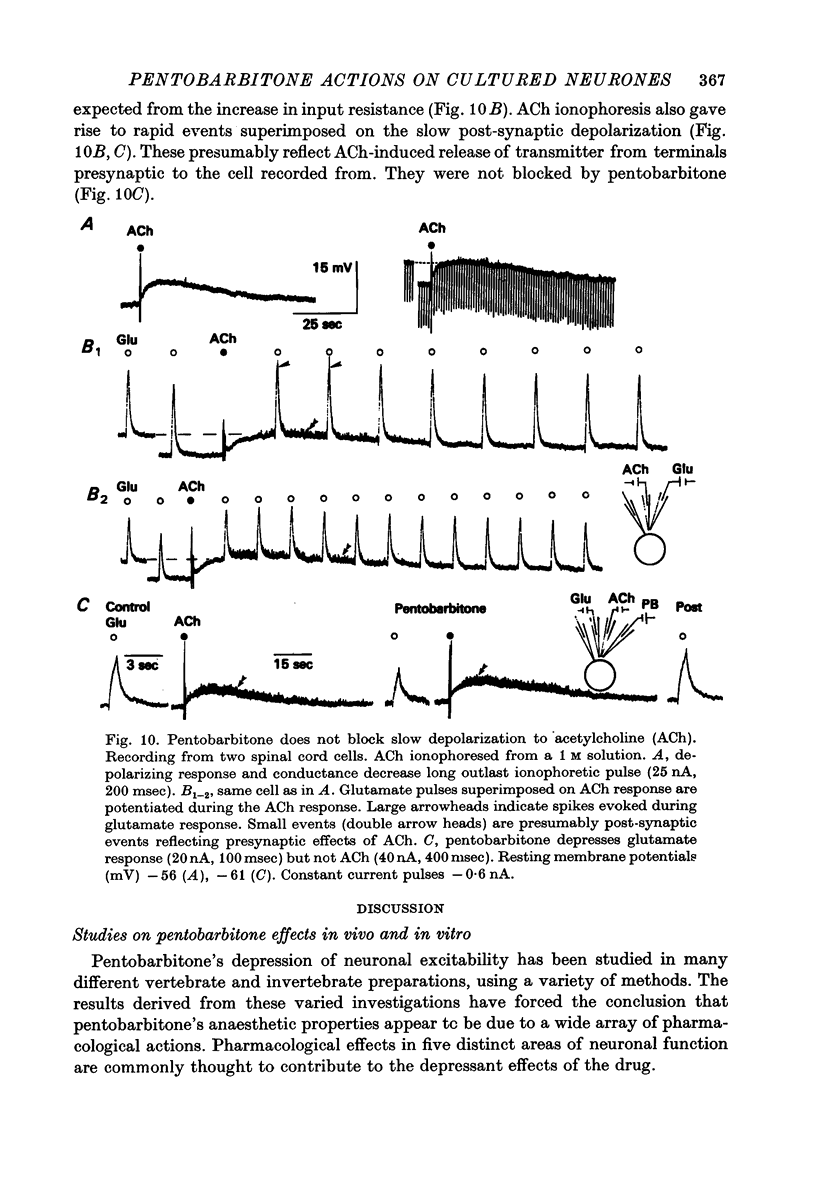
1. The effects of the barbiturate anaesthetic pentobarbitone on the membrane properties and amino acid pharmacology of mammalian C.N.S. neurones grown in tissue culture were studied using intracellular recording coupled with bath application, extracellular ionophoresis, or focal diffusion. 2. The addition of an anaesthetic concentration of pentobarbitone to the bathing medium abolished all spontaneous synaptic activity, but did not render individual cells electrically inexcitable nor prevent evoked synaptic acitivity. 3. Focal ionophoresis of pentobarbitone or diffusion from blunt micropipettes reversibly increased membrane conductance, effectively dampening excitability without directly affecting individual action potential characteristics. 4. Pentobarbitone-induced membrane conductance was reversibly blocked by picrotoxin. The inversion potential of the pentobarbitone voltage response depended on Cl- ion gradients and was similar to that of GABA. 5. Pentobarbitone reversibly enhanced the conductance increase produced by GABA with a variable slowing of response kinetics, shifting GABA dose-response curves to the left. Responses to glycine and beta-alanine were not affected. 6. Higher ionophoretic currents of pentobarbitone, which measurably increased membrane conductance, attenuated and markedly slowed GABA responses. Similar effects on GABA responses were observed by superimposing GABA pulses on low level GABA currents. 7. Pentobarbitone, in the absence of an increase in membrane conductance, reversibly depressed depolarizing responses to glutamate without changing response kinetics. Slower responses to acetylcholine which were associated with an apparent decrease in membrane conductance were not affected by the drug. 8. Analysis of double-reciprocal plot data suggested a non-competitive type of antagonism between pentobarbitone and glutamate. Pentobarbitone depression of glutamate was not affected by picrotoxin. 9. Both GABA and glutamate responses appeared to be equally sensitive to pentobarbitone. Specific interaction of the drug with amino acid receptor-coupled events is indicated by the requirement for pentobarbitone pipette placement close to the amino acid response site. 10. The results suggest that pentobarbitone depresses neuronal excitability by (1) directly activating post-synaptic GABA-receptor coupled Cl- conductance, (2) potentiating post-synaptic GABA-induced conductance events, probably at the level of the GABA receptor, and (3) depressing post-synaptic glutamate-induced excitation, probably at the level of the conductance mechanism.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L. CNS depressants: effects on post-synaptic pharmacology. Brain Res. 1975 Jul 4;92(1):35–55. doi: 10.1016/0006-8993(75)90526-0. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Gainer H. Pentobarbital: selective depression of excitatory postsynaptic potentials. Science. 1973 Nov 16;182(4113):720–722. doi: 10.1126/science.182.4113.720. [DOI] [PubMed] [Google Scholar]
- Barker J. L. Inhibitory and excitatory effects of CNS depressants on invertebrate synapses. Brain Res. 1975 Jul 25;93(1):77–90. doi: 10.1016/0006-8993(75)90287-5. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A. Gamma-aminobutyric acid: role in primary afferent depolarization. Science. 1972 Jun 2;176(4038):1043–1045. doi: 10.1126/science.176.4038.1043. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A., Padjen A. Studies on convulsants in the isolated frog spinal cord. I. Antagonism of amino acid responses. J Physiol. 1975 Mar;245(3):521–536. doi: 10.1113/jphysiol.1975.sp010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A., Padjen A. Studies on convulsants in the isolated frog spinal cord. II. Effects on root potentials. J Physiol. 1975 Mar;245(3):537–548. doi: 10.1113/jphysiol.1975.sp010860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A. The pharmacology and ionic dependency of amino acid responses in the frog spinal cord. J Physiol. 1973 Jan;228(2):259–277. doi: 10.1113/jphysiol.1973.sp010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. Barbiturates block calcium uptake by stimulated and potassium-depolarized rat sympathetic ganglia. J Pharmacol Exp Ther. 1976 Jan;196(1):80–86. [PubMed] [Google Scholar]
- Blaustein M. P. Barbiturates block sodium and potassium conductance increases in voltage-clamped lobster axons. J Gen Physiol. 1968 Mar;51(3):293–307. doi: 10.1085/jgp.51.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Ector A. C. Barbiturate inhibition of calcium uptake by depolarized nerve terminals in vitro. Mol Pharmacol. 1975 May;11(3):369–378. [PubMed] [Google Scholar]
- Bowery N. G., Dray A. Barbiturate reversal of amino acid antagonism produced by convulsant agents. Nature. 1976 Nov 18;264(5583):276–278. doi: 10.1038/264276a0. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A. Gamma-aminobutyric acid antagonism and presynaptic inhibition in the frog spinal cord. Science. 1972 Jan 21;175(4019):331–333. doi: 10.1126/science.175.4019.331. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo A. Procaine, pentobarbital and halothane: effects on the mammalian myoneural junction. J Pharmacol Exp Ther. 1971 May;177(2):360–368. [PubMed] [Google Scholar]
- Haycock J. W., Levy W. B., Cotman C. W. Pentobarbital depression of stimulus-secretion coupling in brain--selective inhibition of depolarization-induced calcium-dependent release. Biochem Pharmacol. 1977 Jan 15;26(2):159–161. doi: 10.1016/0006-2952(77)90389-6. [DOI] [PubMed] [Google Scholar]
- Holmes J. C., Schneider F. H. Pentobarbitone inhibition of catecholamine secretion. Br J Pharmacol. 1973 Oct;49(2):205–213. doi: 10.1111/j.1476-5381.1973.tb08366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARRABEE M. G., POSTERNAK J. M. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J Neurophysiol. 1952 Mar;15(2):91–114. doi: 10.1152/jn.1952.15.2.91. [DOI] [PubMed] [Google Scholar]
- LOYNING Y., OSHIMA T., YOKOTA T. SITE OF ACTION OF THIAMYLAL SODIUM ON THE MONOSYNAPTIC SPINAL REFLEX PATHWAY IN CATS. J Neurophysiol. 1964 May;27:408–428. doi: 10.1152/jn.1964.27.3.408. [DOI] [PubMed] [Google Scholar]
- Larson M. D., Major M. A. The effect of hexobarbital on the duration of the recurrent IPSP in cat motoneurons. Brain Res. 1970 Jul 14;21(2):309–311. doi: 10.1016/0006-8993(70)90377-x. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Frazier D. T., Deguchi T., Cleaves C. A., Ernau M. C. The active form of pentobarbital in squid giant axons. J Pharmacol Exp Ther. 1971 Apr;177(1):25–33. [PubMed] [Google Scholar]
- Nicoll R. A., Eccles J. C., Oshima T., Rubia F. Prolongation of hippocampal inhibitory postsynaptic potentials by barbiturates. Nature. 1975 Dec 18;258(5536):625–627. doi: 10.1038/258625a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Padjen A., Barker J. L. Analysis of amino acid responses on frog motoneurones. Neuropharmacology. 1976 Jan;15(1):45–53. doi: 10.1016/0028-3908(76)90096-4. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Pentobarbital: action on frog motoneurons. Brain Res. 1975 Oct 10;96(1):119–123. doi: 10.1016/0006-8993(75)90582-x. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Presynaptic action of barbiturates in the frog spinal cord. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1460–1463. doi: 10.1073/pnas.72.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A. The effects of anaesthetics on synaptic excitation and inhibition in the olfactory bulb. J Physiol. 1972 Jun;223(3):803–814. doi: 10.1113/jphysiol.1972.sp009875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W., Lamar E. E., Bayless J. D. Calcium-induced release of gamma-aminobutyric acid from synaptosomes: effects of tranquilizer drugs. J Neurochem. 1977 Feb;28(2):299–305. doi: 10.1111/j.1471-4159.1977.tb07748.x. [DOI] [PubMed] [Google Scholar]
- Peck E. J., Miller A. L., Lester B. R. Pentobarbital and synaptic high-affinity receptive sites for gamma-aminobutyric acid. Brain Res Bull. 1976 Nov-Dec;1(6):595–597. doi: 10.1016/0361-9230(76)90087-3. [DOI] [PubMed] [Google Scholar]
- Peper K., Dreyer F., Müller K. D. Analysis of cooperativity of drug-receptor interaction by quantitative iontophoresis at frog motor end plates. Cold Spring Harb Symp Quant Biol. 1976;40:187–192. doi: 10.1101/sqb.1976.040.01.020. [DOI] [PubMed] [Google Scholar]
- Proctor W. R., Weakly J. N. A comparison of the presynaptic and post-synaptic actions of pentobarbitone and phenobarbitone in the neuromuscular junction of the frog. J Physiol. 1976 Jun;258(1):257–268. doi: 10.1113/jphysiol.1976.sp011418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom B. R., Barker J. L., Nelson P. G. Two mechanisms for poststimulus hyperpolarisations in cultured mammalian neurones. Nature. 1975 Jul 31;256(5516):424–425. doi: 10.1038/256424a0. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Barker J. L. Pentobarbital modulates transmitter effects on mouse spinal neurones grown in tissue culture. Nature. 1975 Apr 24;254(5502):703–705. doi: 10.1038/254703a0. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Barker J. L. Pentobarbital selectively enhances GABA-mediated post-synaptic inhibition in tissue cultured mouse spinal neurons. Brain Res. 1976 Sep 24;114(3):530–535. doi: 10.1016/0006-8993(76)90977-x. [DOI] [PubMed] [Google Scholar]
- Richards C. D. On the mechanism of barbiturate anaesthesia. J Physiol. 1972 Dec;227(3):749–767. doi: 10.1113/jphysiol.1972.sp010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D., Smaje J. C. Anaesthetics depress the sensitivity of cortical neurones to L-glutamate. Br J Pharmacol. 1976 Nov;58(3):347–357. [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT R. F. PHARMACOLOGICAL STUDIES ON THE PRIMARY AFFERENT DEPOLARIZATION OF THE TOAD SPINAL CORD. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963 Jul 2;277:325–346. doi: 10.1007/BF00362515. [DOI] [PubMed] [Google Scholar]
- SOMJEN G. G. Effects of ether and thiopental on spinal presynaptic terminals. J Pharmacol Exp Ther. 1963 Jun;140:396–402. [PubMed] [Google Scholar]
- Seyama I., Narahashi T. Mechanism of blockade of neuromuscular transmission by pentobarbital. J Pharmacol Exp Ther. 1975 Jan;192(1):95–104. [PubMed] [Google Scholar]
- Staiman A., Seeman P. The impulse-blocking concentrations of anesthetics, alcohols, anticonvulsants, barbiturates, and narcotics on phrenic and sciatic nerves. Can J Physiol Pharmacol. 1974 Jun;52(3):535–550. doi: 10.1139/y74-071. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. The effect of anesthetic agents on skeletal muscle membrane. Acta Physiol Scand. 1956 Nov 5;37(4):335–349. doi: 10.1111/j.1748-1716.1956.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Thomson T. D., Turkanis S. A. Barbiturate-induced transmitter release at a frog neuromuscular junction. Br J Pharmacol. 1973 May;48(1):48–58. doi: 10.1111/j.1476-5381.1973.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weakly J. N. Effect of barbiturates on 'quantal' synaptic transmission in spinal motoneurones. J Physiol. 1969 Sep;204(1):63–77. doi: 10.1113/jphysiol.1969.sp008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland B. F., Ward D., Johns T. R. The effect of methohexital at the neuromuscular junction. Brain Res. 1971 Mar 5;26(2):465–468. [PubMed] [Google Scholar]




