Abstract
Chlamydia pneumoniae has been shown to accelerate atherosclerotic lesion development in hyperlipidemic animals. This study showed that C. pneumoniae did not accelerate lesion development in mice if a high-fat/high-cholesterol diet was started after infection, indicating that C. pneumoniae is a co-risk factor with hyperlipidemia for cardiovascular disease.
The association of Chlamydia pneumoniae and coronary heart disease was first demonstrated by seroepidemiological studies (17) followed by detection of C. pneumoniae in atherosclerotic lesions (8). Experimental studies suggest that C. pneumoniae is a co-risk factor with hyperlipidemia for atherosclerosis. In genetically and diet-induced hyperlipidemic mice, C. pneumoniae accelerates formation of preexisting lesions (4, 12, 16) and plaque development when C. pneumoniae and a high-fat/high-cholesterol diet are simultaneously administered (2, 7, 14). However, this is still controversial as other reports have not observed this acceleration (1, 5). Methodological differences may explain the discrepancies (16; C. C. Kuo, L. A. Campbell, and M. E. Rosenfeld, Letter, Circulation 105:e34, 2000). Without hyperlipidemia, C. pneumoniae causes only inflammatory changes in the heart and aorta of mice and rabbits but does not initiate formation of atherosclerotic lesions (3, 6, 7, 10). It has not yet been determined whether C. pneumoniae promotes atherogenesis if hyperlipidemia is induced after infection. In the present study, we investigated whether C. pneumoniae infection prior to induction of hyperlipidemia accelerates the development of atherosclerosis in the aortic sinus of C57BL/6J mice.
Eight-week-old pathogen-free male C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Mice were kept under modified pathogen-free conditions in filter-top cages. Mice were sedated by intraperitoneal injections of ketamine and xylazine (Fort Dodge Laboratories and Lloyd Laboratories, Shenandoah, Iowa) and inoculated intranasally with 3 × 107 inclusion-forming units of purified organisms of C. pneumoniae (strain AR-39) according to our previously described protocol (3). Control mice were sham inoculated with sterile phosphate-buffered saline. Mice were initially fed a regular chow diet and then switched to an atherogenic diet consisting of 15% fat, 1.25% cholesterol, and 0.5% sodium cholate (TD 88051; Harlan Teklad, Madison, Wis.) starting at either 5 (group 1) or 7 (group 2) weeks after the first inoculation (Fig. 1). This time frame was chosen according to a previous study where residual inflammatory changes in hearts and aorta were observed up to 8 weeks after infection (3). The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Washington.
FIG. 1.
Shown is the experimental design regarding the time sequence of inoculation, the start of the high-fat/high-cholesterol diet, and examination.
Animals were sacrificed after 15 or 17 weeks on the atherogenic diet, at which time fatty streaks had formed in the aortic sinus (15). The animals were perfusion fixed with 10% buffered formalin administered through the left ventricle. The lungs, heart, and thoracic aorta were removed intact. The base of each heart was frozen in OCT medium (Sakura Finetek, Torrance, Calif.) and cryosectioned as described by Paigen (15). For quantification of the average area of fatty streaks, the cross-sectional area of oil red O staining was measured in 15-step sections from the points of emergence to disappearance of the aortic valves by using computer-assisted morphometry (Image-Pro Plus Software; Media Cybernetics, Silver Spring, Md.). Measurements were done in a blinded fashion. The unpaired Student t test was used for statistical analysis.
Detection of C. pneumoniae in the aortic tissues was done by immunohistochemistry and PCR (3). Plasma was separated from heparinized blood and frozen at −70°C for serology and lipid measurements. C. pneumoniae-specific antibody titers were determined by the micro-immunofluorescence test (3). Total blood cholesterol and triglycerides were measured using a commercial enzymatic test kit (Sigma, St. Louis, Mo.).
Clinical signs in the infected mice included increased respiratory rate and some nasal and ocular discharge. No mortality was observed. No significant difference in body weights or plasma lipids were noted between the infected and control animals at the time of sacrifice (Table 1). All of the infected mice developed immunoglobulin G (IgG) antibody and all of the control mice remained antibody negative.
TABLE 1.
Body weights, antibody titers, and lipid profiles of C57BL/6J mice inoculated with C. pneumoniae in comparison to control mice
| Group (no. of weeks after first inoculation)a | Infection | Body weight (g [mean ± SD]) | Antibody titer (range) | Cholesterol (mg/dl [mean ± SD]) | Triglycerides (mg/dl [mean ± SD]) |
|---|---|---|---|---|---|
| Group 1 (5 weeks) | Yes (n = 15) | 31.7 ± 2.7 | 1:128-1:1024 | 140.9 ± 34.7 | 49.0 ± 12.9 |
| No (n = 13) | 30.6 ± 1.5 | Negative | 157.3 ± 25.3 | 45.6 ± 21.6 | |
| Group 2 (7 weeks) | Yes (n = 14) | 30.3 ± 1.3 | 1:64-1:512 | 158.4 ± 21.5 | 46.5 ± 15.6 |
| No (n = 15) | 30.8 ± 1.4 | Negative | 165.2 ± 33.7 | 37.5 ± 11.4 |
For each group, the number of weeks is the time after the first inoculation that the atherogenic diet was begun.
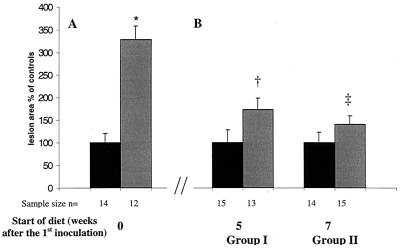
The average area of atherosclerotic lesions in the aortic sinus was not significantly greater for infected animals than for control mice when the animals were placed on the diet at 5 weeks postinoculation (4,027 ± 1,034 μm2 versus 2,337 ± 649 μm2; P = 0.15) or 7 weeks postinoculation (2,630 ± 473 μm2 versus 1,865 ± 423 μm2; P = 0.22) (Fig. 2B). In a previous study, we demonstrated that when the atherogenic diet was administered simultaneously with the first infection, the lesion size was 3.3-fold greater (P < 0.02) in infected versus control mice (2) (Fig. 2A).
FIG. 2.
Shown are the sizes of fatty streaks in the aortic sinus when the high-fat/high-cholesterol diet was started simultaneously with infection (A) in comparison to when the high-fat/high-cholesterol diet was given after the infection (B). (Panel A is reprinted from reference 2 with permission of the publisher.) Average lesion area of infected mice (grey bars) was normalized to that of control mice (black bars = 100%). Bar graphs indicate means ± standard error of the mean. (∗, P = 0.015 versus control; †, P = 0.15 versus control; ‡, P = 0.22 versus control).
C. pneumoniae was detected in the thoracic aorta by PCR for only 1 out of 29 infected animals. PCR was negative for noninfected controls. Immunohistochemistry was negative for the aortic sinuses in 12 infected animals selectively tested.
This study showed that infection with C. pneumoniae prior to establishment of stable hyperlipidemia did not accelerate the progression of atherosclerosis, which may be due to the failure of C. pneumoniae to establish persistent infection in the aorta. In normolipidemic mice, C. pneumoniae disseminates from the lungs to the aorta but infection in the aorta is transient (13) and mice do not develop atherosclerosis (3). In the presence of stable hyperlipidemia and established atheromatous lesions, C. pneumoniae establishes persistent infection (13). It has been postulated that the ability of C. pneumoniae to establish persistent infection in the atherosclerotic lesions is an important factor for C. pneumoniae to effect atherogenesis.
In this study, an atherogenic diet was started at 1 or 3 weeks following the third intranasal inoculation. Although not statistically significant, the average area of fatty streak was greater in infected than in uninfected mice at both time points. The difference was greater if the atherogenic diet was started at 1 week than 3 weeks after the last inoculation. These findings suggest that the additive effect of C. pneumoniae and hyperlipidemia on induction and progression of atherosclerosis may depend on the severity of residual inflammatory changes in the aorta from C. pneumoniae infection (3) and the level of hyperlipidemia, because the stable plasma lipid level is not established until after 4 weeks of feeding mice an atherogenic diet (11).
In animal experiments, it has been shown that multiple infections with C. pneumoniae have a greater effect on atherosclerotic lesion development than a single infection in hyperlipidemic animals (1, 2, 4, 5, 7, 12, 14, 16) and that the effects were specific to C. pneumoniae, as infection with C. trachomatis did not show the acceleration of lesion development (7).
From a clinical point of view, the present study may add valuable information to our understanding of the role of C. pneumoniae in cardiovascular disease. In humans, C. pneumoniae infections occur early in childhood, when hyperlipidemia and atherosclerotic lesions generally have not yet developed. Seroepidemiologic studies have shown that everyone is infected between the ages of 5 and 14 years and reinfected throughout life (9). The antibody prevalence in the adult population is 50%. Interestingly, C. pneumoniae is detected in atherosclerotic tissues in 50% of adults (8). However, in a study by Taylor-Robinson et al., C. pneumoniae was not detected in vascular tissues in persons younger than 15 years of age (18). The data suggest that C. pneumoniae infections have a more profound effect on atherogenesis in adults, where hyperlipidemia is prevalent and atherosclerosis is ubiquitous.
In conclusion, the present study further supports the hypothesis that C. pneumoniae is not an independent risk factor but acts in concert with hyperlipidemia to exacerbate atherosclerotic lesion formation.
Acknowledgments
This study was supported by National Institutes of Health grant HL-56036.
We thank Anne Tecklenburg, June Zhang, and Anne Nguyen for technical assistance.
Editor: D. L. Burns
REFERENCES
- 1.Aalto-Setala, K., K. Laitinen, L. Erkkila, M. Leinonen, M. Jauhiainen, C. Ehnholm, M. Tamminen, M. Puolakkainen, I. Pentila, and P. Saikku. 2001. Chlamydia pneumoniae does not increase atherosclerosis in the aortic root of apolipoprotein E-deficient mice. Arteroscler. Thromb. Vasc. Biol. 21:578-584. [DOI] [PubMed] [Google Scholar]
- 2.Blessing, E., L. A. Campbell, M. E. Rosenfeld, N. Cough, and C. C. Kuo. 2001. Chlamydia pneumoniae infection accelerates hyperlipidemia induced atherosclerotic lesion development in C57BL/6J mice. Atherosclerosis 158:13-17. [DOI] [PubMed] [Google Scholar]
- 3.Blessing, E., T. M. Lin, L. A. Campbell, M. E. Rosenfeld, D. Lloyd, and C. C. Kuo. 2000. Chlamydia pneumoniae induces inflammatory changes in the heart and aorta of normocholesterolemic C57BL/6J mice. Infect. Immun. 68:4765-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnet, M. S., C. A. Gaydos, G. E. Madico, S. M. Glad, B. Paigen, T. C. Quinn, and S. E. Epstein. 2001. Atherosclerosis in apoE knockout mice infected with multiple pathogens. J. Infect. Dis. 183:226-231. [DOI] [PubMed] [Google Scholar]
- 5.Caliguri, G., M. Rottenberg, A. Nicoletti, H. Wigzell, and G. Hansson. 2001. Chlamydia pneumoniae infection does not induce or modify atherosclerosis in mice. Circulation 103:2834-2838. [DOI] [PubMed] [Google Scholar]
- 6.Fong, I. W., B. Chiu, E. Viira, D. Jang, and J. B. Mahony. 1999. De novo induction of atherosclerosis by Chlamydia pneumoniae in a rabbit model. Infect. Immun. 11:6048-6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu, H., G. N. Pierce, and G. Zhong. 1999. The atherogenic effects of chlamydia are dependent on serum cholesterol and specific to Chlamydia pneumoniae. J. Clin. Investig. 103:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo, C. C., and L. A. Campbell. 2000. Detection of Chlamydia pneumoniae in arterial tissues. J. Infect. Dis. 81(Suppl. 3):S432-S436. [DOI] [PubMed] [Google Scholar]
- 9.Kuo, C. C., L. A. Jackson, L. A. Campbell, and J. T. Grayston. 1995. Chlamydia pneumoniae (TWAR). Clin. Microbiol Rev. 8:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laitinen, K., A. Laurila, L. Pyhala, M. Leinonen, and P. Saikku. 1997. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect. Immun. 65:4832-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeBoeuf, R. C., and E. A. Kirk. 1996. Dietary regulation of plasma lipid concentration in the mouse, p. 316-330. In G. A. Bray and D. H. Ryan (ed.), Nutrition, genetics, and heart disease, vol. 6. Pennington Center Nutrition Series. Louisiana State University Press, Baton Rouge, La.
- 12.Moazed, T., L. A. Campbell, M. E. Rosenfeld, J. T. Grayston, and C. C. Kuo. 1999. Chlamydia pneumoniae infection accelerates the progession of atherosclerosis in apolipoprotein E-deficient mice. J. Infect. Dis. 180:238-241. [DOI] [PubMed] [Google Scholar]
- 13.Moazed, T., C. C. Kuo, J. T. Grayston, and L. A. Campbell. 1997. Murine models of Chlamydia pneumoniae infection and atherosclerosis. J. Infect. Dis. 175:883-890. [DOI] [PubMed] [Google Scholar]
- 14.Muhlestein, J. B., J. L. Anderson, E. H. Hammond, L. Zhao, S. Trehan, E. Schwobe, and J. F. Carlquist. 1998. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation 97:633-636. [DOI] [PubMed] [Google Scholar]
- 15.Paigen, B., A. Morrow, P. A. Holmes, D. Mitchel, and R. A. Williams. 1987. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis 68:231-240. [DOI] [PubMed] [Google Scholar]
- 16.Rothstein, N. M., T. C. Quinn, G. Madico, C. A. Gaydos, and C. J. Lowenstein. 2001. Effect of azithromycin on murine arteriosclerosis exacerbated by Chlamydia pneumoniae. J. Infect. Dis. 183:232-238. [DOI] [PubMed] [Google Scholar]
- 17.Saikku, P., M. Leinonen, K. Mattila, M. R. Ekman, M. S. Nieminen, P. H. Makela, J. K. Huttunen, and V. Valtonen. 1988. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet 2:983-986. [DOI] [PubMed] [Google Scholar]
- 18.Taylor-Robinson, D., G. Ong, B. J. Thomas, M. L. Rose, and M. H. Yacoub. 1998. Chlamydia pneumoniae in vascular tissues from heart-transplant donors. Lancet 351:1255.. [DOI] [PubMed] [Google Scholar]