Abstract
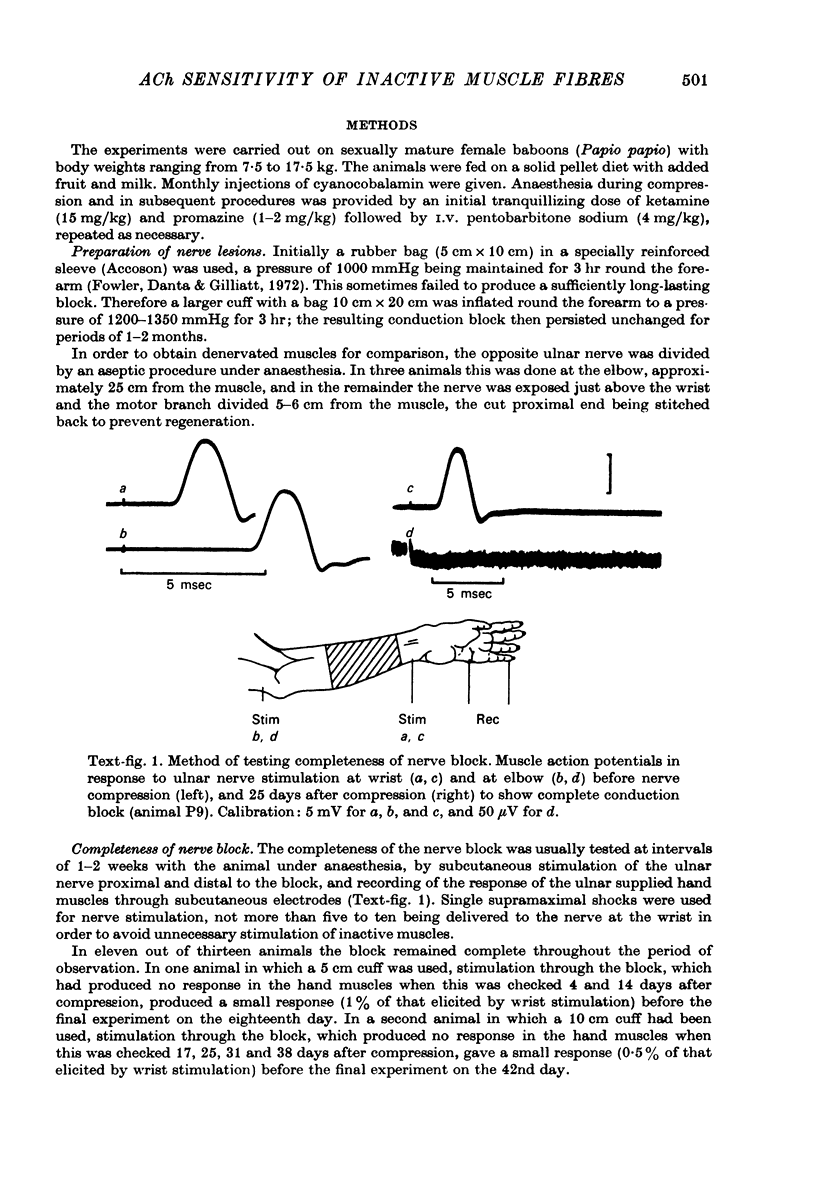
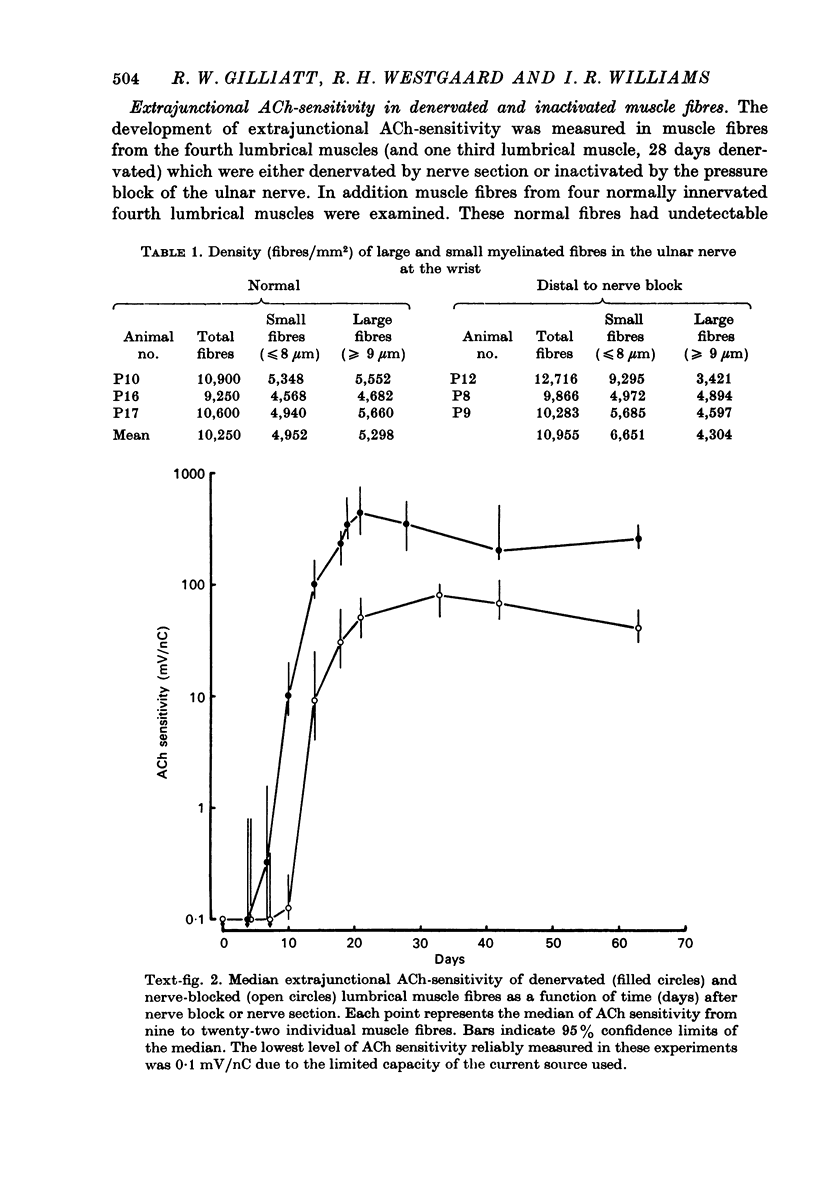
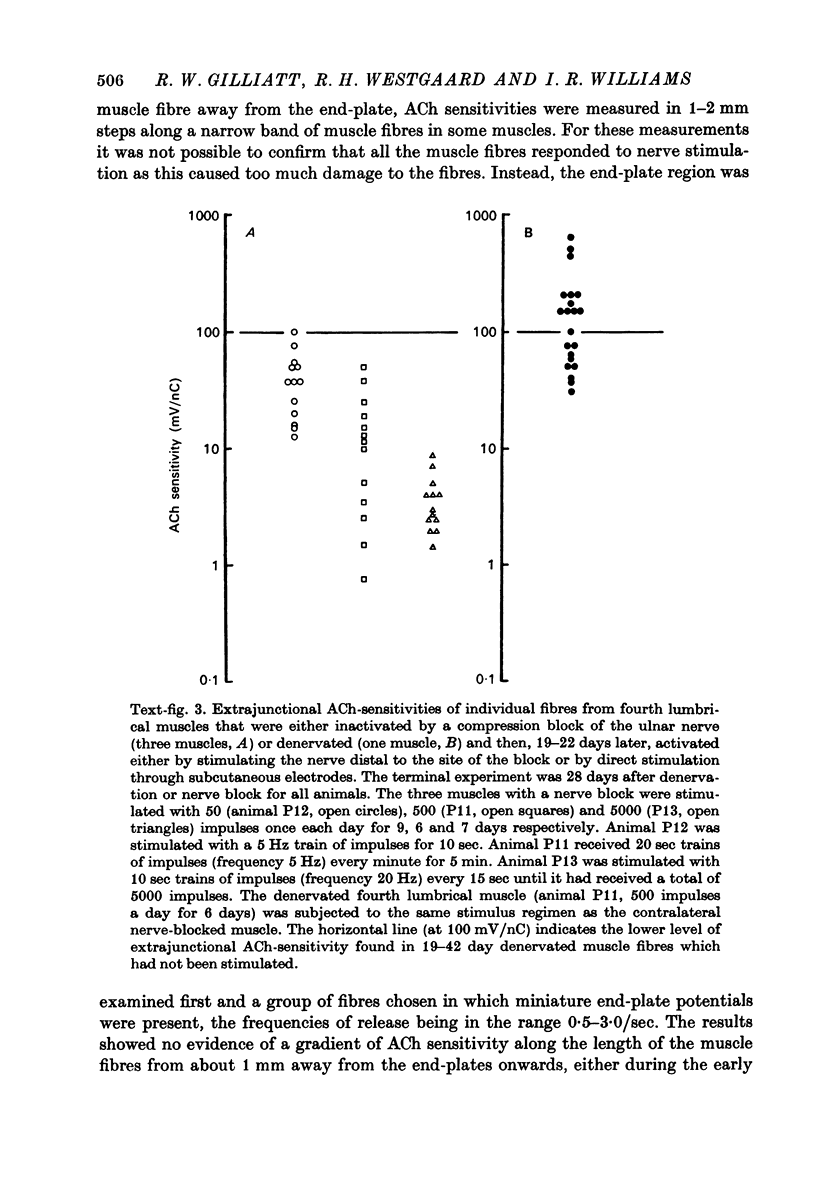
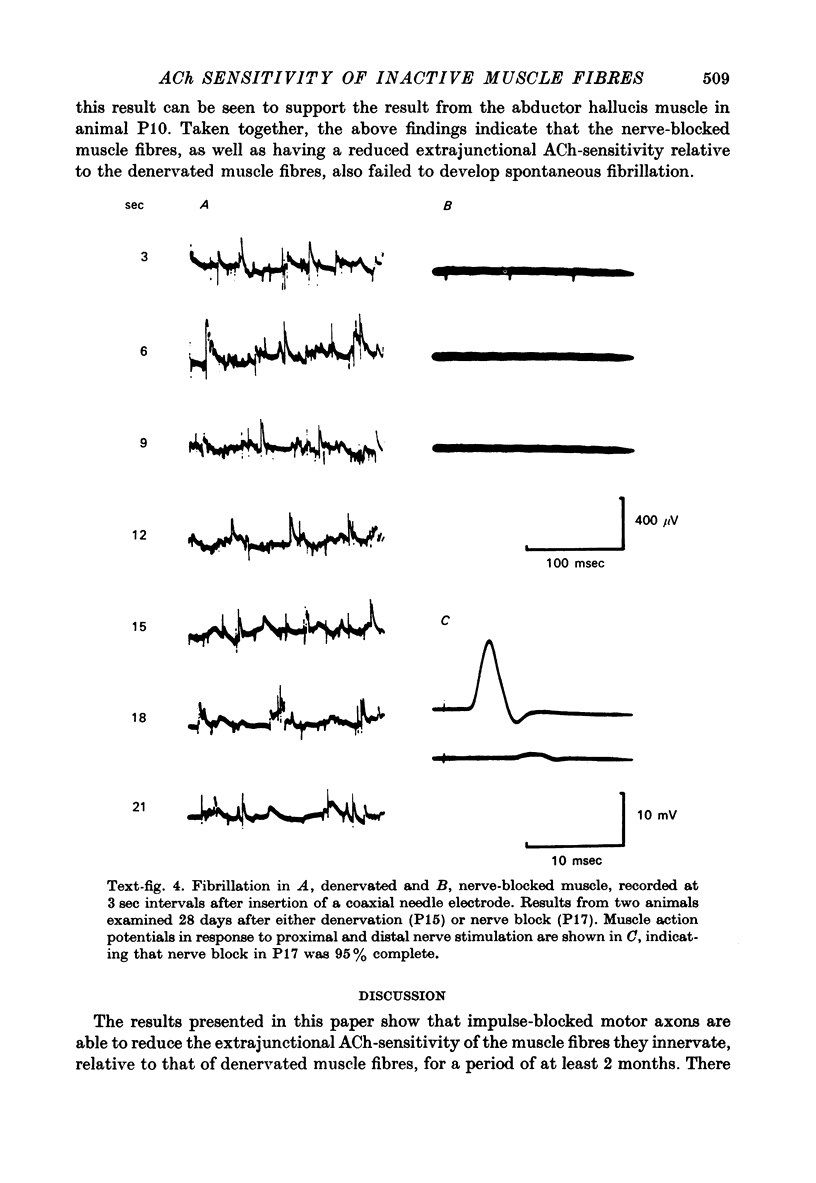
1. Nerve-evoked muscular activity was abolished in the small hand muscles of the baboon for 1-2 months by a 3 hr period of nerve compression from a pneumatic tourniquet inflated round the forearm. In the large diameter nerve fibres, this produced either a prolonged conduction block due to local myelin damage at the site of compression, or (in 10-30% of the large fibres) Wallerian degeneration. 2. At varying intervals after nerve compression the extrajunctional acetylcholine (ACh)-sensitivity of innervated but inactive muscle fibres in the fourth lumbrical muscle was measured. Observations were also made on lumbrical muscle fibres at similar intervals after surgical denervation. 3. The ACh sensitivity of nerve-blocked muscle fibres started to develop later than in denervated muscle fibres (10 vs. 7 days) and remained at a lower level (40-80 mV/nC, median ACh-sensitivity) than that of denervated muscle fibres (200-437 mV/nC) from 21 to 63 days after nerve block or denervation. 4. In stimulation experiments on four muscles, extrajunctional ACh-sensitivity of both denervated and innervated but inactive fourth lumbrical muscle fibres was reduced by muscular activity. 5. In four animals mild compression was used in the lower limb to produce persistent nerve block without Wallerian degeneration. With one exception (in which some Wallerian degeneration had occurred) recording with a co-axial needle from abductor hallucis showed no spontaneous fibrillation up to 28 days after compression, although the extrajunctional ACh-sensitivity of the muscle fibres appeared to reach levels similar to those observed in the forelimb. All four muscles developed a slight increase in insertion activity after 1-2 weeks. 6. It may be concluded that both muscular activity and some other neural influence, independent of muscular activity, are able to influence extrajunctional muscle properties in the baboon. The neural influence appears to be more effective in preventing spontaneous fibrillation than in preventing a rise in ACh sensitivity of the extrajunctional muscle membrane.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Schuh F. T., Kauffman F. C. Early membrane depolarization of the fast mammalian muscle after denervation. Pflugers Arch. 1971;328(1):36–50. doi: 10.1007/BF00587359. [DOI] [PubMed] [Google Scholar]
- Bray J. J., Harris A. J. Dissociation between nerve-muscle transmission and nerve trophic effects on rat diaphragm using type D botulinum toxin. J Physiol. 1975 Dec;253(1):53–77. doi: 10.1113/jphysiol.1975.sp011179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano A., Lutzemberger L., Nicotra L. Non-equivalence of impulse blockade and denervation in the production of membrane changes in rat skeletal muscle. J Physiol. 1977 Dec;273(3):691–706. doi: 10.1113/jphysiol.1977.sp012117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande S. S., Albuquerque E. X., Guth L. Neurotrophic regulation of prejunctional and postjunctional membrane at the mammalian motor endplate. Exp Neurol. 1976 Oct;53(1):151–165. doi: 10.1016/0014-4886(76)90289-2. [DOI] [PubMed] [Google Scholar]
- Drachman D. B., Witzke F. Trophic regulation of acetylcholine sensitivity of muscle: effect of electrical stimulation. Science. 1972 May 5;176(4034):514–516. doi: 10.1126/science.176.4034.514. [DOI] [PubMed] [Google Scholar]
- EMMELIN N., MALM L. DEVELOPMENT OF SUPERSENSITIVITY AS DEPENDENT ON THE LENGTH OF DEGENERATING NERVE FIBRES. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:142–145. doi: 10.1113/expphysiol.1965.sp001776. [DOI] [PubMed] [Google Scholar]
- Fowler T. J., Danta G., Gilliatt R. W. Recovery of nerve conduction after a pneumatic tourniquet: observations on the hind-limb of the baboon. J Neurol Neurosurg Psychiatry. 1972 Oct;35(5):638–647. doi: 10.1136/jnnp.35.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliatt R. W., McDonald W. I., Rudge P. Proceeding: The site of conduction block in peripheral nerves compressed by a pneumatic tourniquet. J Physiol. 1974 Apr;238(1):31P–32P. [PubMed] [Google Scholar]
- Gilliatt R. W., Westgaard R. H., Williams I. R. Acetylcholine-sensitivity of denervated and inactivated baboon muscle fibres [proceedings]. J Physiol. 1977 Oct;271(2):21P–22P. [PubMed] [Google Scholar]
- Guth L. "Trophic" influences of nerve on muscle. Physiol Rev. 1968 Oct;48(4):645–687. doi: 10.1152/physrev.1968.48.4.645. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Thesleff S. Nerve stump length and membrane changes in denervated skeletal muscle. Nat New Biol. 1972 Mar 15;236(63):60–61. doi: 10.1038/newbio236060a0. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Lomo T., Nicolaysen K., Westgaard R. H. Hyperinnervation of skeletal muscle fibers: dependence on muscle activity. Science. 1973 Aug 10;181(4099):559–561. doi: 10.1126/science.181.4099.559. [DOI] [PubMed] [Google Scholar]
- Jones R., Vrbová G. Two factors responsible for the development of denervation hypersensitivity. J Physiol. 1974 Feb;236(3):517–538. doi: 10.1113/jphysiol.1974.sp010450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie P. A., Collier B., Tenehouse A. Comparison of alpha-bungarotoxin binding to skeletal muscles after inactivity or denervation. Nature. 1976 Mar 25;260(5549):349–350. doi: 10.1038/260349a0. [DOI] [PubMed] [Google Scholar]
- Lavoie P. A., Collier B., Tenenhouse A. Role of skeletal muscle activity in the control of muscle acetylcholine sensitivity. Exp Neurol. 1977 Jan;54(1):148–171. doi: 10.1016/0014-4886(77)90242-4. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Control of ACh sensitivity in rat muscle fibers. Cold Spring Harb Symp Quant Biol. 1976;40:263–274. doi: 10.1101/sqb.1976.040.01.027. [DOI] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Further studies on the control of ACh sensitivity by muscle activity in the rat. J Physiol. 1975 Nov;252(3):603–626. doi: 10.1113/jphysiol.1975.sp011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Ochoa J., Fowler T. J., Gilliatt R. W. Anatomical changes in peripheral nerves compressed by a pneumatic tourniquet. J Anat. 1972 Dec;113(Pt 3):433–455. [PMC free article] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B., Griffin J. W. Effect of muscle disuse on acetylcholine receptors. Nature. 1976 Mar 25;260(5549):352–353. doi: 10.1038/260352a0. [DOI] [PubMed] [Google Scholar]
- Purves D., Sakmann B. Membrane properties underlying spontaneous activity of denervated muscle fibres. J Physiol. 1974 May;239(1):125–153. doi: 10.1113/jphysiol.1974.sp010559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Sakmann B. The effect of contractile activity on fibrillation and extrajunctional acetylcholine-sensitivity in rat muscle maintained in organ culture. J Physiol. 1974 Feb;237(1):157–182. doi: 10.1113/jphysiol.1974.sp010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge P. Tourniquet paralysis with prolonged conduction block. An electro-physiological study. J Bone Joint Surg Br. 1974 Nov;56-B(4):716–720. doi: 10.1302/0301-620X.56B4.716. [DOI] [PubMed] [Google Scholar]
- Thesleff S., Ward M. R. Studies on the mechanism of fibrillation potentials in denervated muscle. J Physiol. 1975 Jan;244(2):313–323. doi: 10.1113/jphysiol.1975.sp010800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojaborg W. Prolonged conduction block with axonal degeneration. An electrophysiological study. J Neurol Neurosurg Psychiatry. 1977 Jan;40(1):50–57. doi: 10.1136/jnnp.40.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnick J. E., Albuquerque E. X., Guth L. The demonstration of neurotrophic function by application of colchicine or vinblastine to the peripheral nerve. Exp Neurol. 1977 Nov;57(2):622–636. doi: 10.1016/0014-4886(77)90094-2. [DOI] [PubMed] [Google Scholar]
- Westgaard R. H. Influence of activity on the passive electrical properties of denervated soleus muscle fibres in the rat. J Physiol. 1975 Oct;251(3):683–697. doi: 10.1113/jphysiol.1975.sp011116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams I. R., Gilliatt R. W. Regeneration distal to a prolonged conduction block. J Neurol Sci. 1977 Aug;33(1-2):267–273. doi: 10.1016/0022-510x(77)90199-x. [DOI] [PubMed] [Google Scholar]




