Abstract
1. The effect of patterned preganglionic stimulation on acetylcholine (ACh) stores was studied in the superior cervical ganglion of the cat. 2. Patterns of stimulation based on 0.5 and 1.0 s trains at frequencies from 10 to 80/s and intervals between trains of 2-180 s increased ACh stores, over periods of 1-3 h stimulation, to the same extent (30-70%) as found earlier to result from continuous stimulation. 3. If the mean frequency of the overall pattern was greater than 5/s the increase in stores developed after termination of the stimulation. If the mean frequency was less than 5/s the increase developed progressively during stimulation and it was then maintained without accommodation over 7 h of stimulation. 4. The magnitude of the increase in ACh stores was linearly related to the frequency within the trains from 10 to 40/s, decreasing with further increase in frequency, and absent below 6-7/s. 5. The ACh stores were increased by patterns of stimulation that mimicked discharging of preganglionic neurones driven by the central respiratory drive under hypercapnic or asphyxial stress; and were increased similarly in animals respired with hypercapnic or partially asphyxial gas mixtures when the preganglionic innervation was intact, but not when it was severed. 6. The variance between ACh stores of ganglia from different animals was reduced in animals held in a controlled environment for 1-2 weeks before experimentation. 7. It is concluded that patterning of preganglionic inputs into high frequency trains is a code for the ongoing modulation of transmitter stores in a sympathetic ganglion. 8. The findings are discussed in relation to current knowledge of ganglionic transmission and to after-hyperpolarization in mammalian unmyelinated nerve fibres.
Full text
PDF

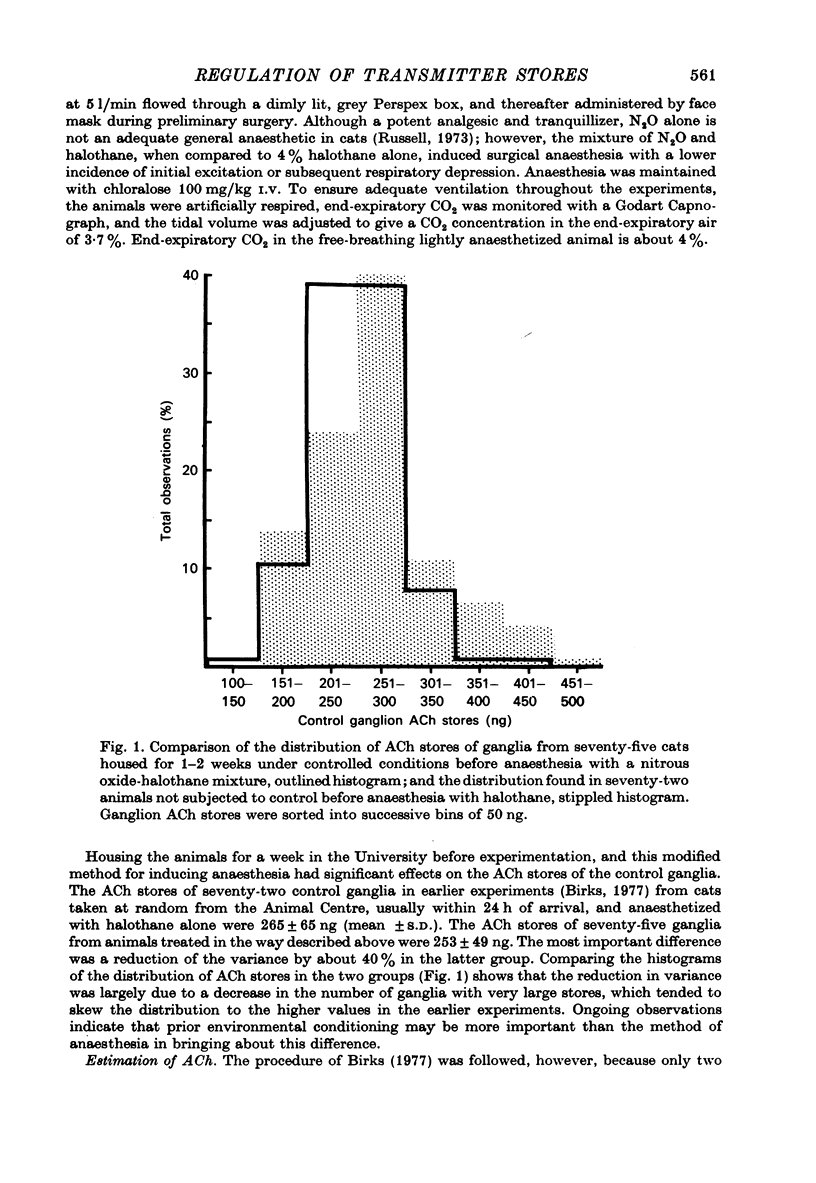

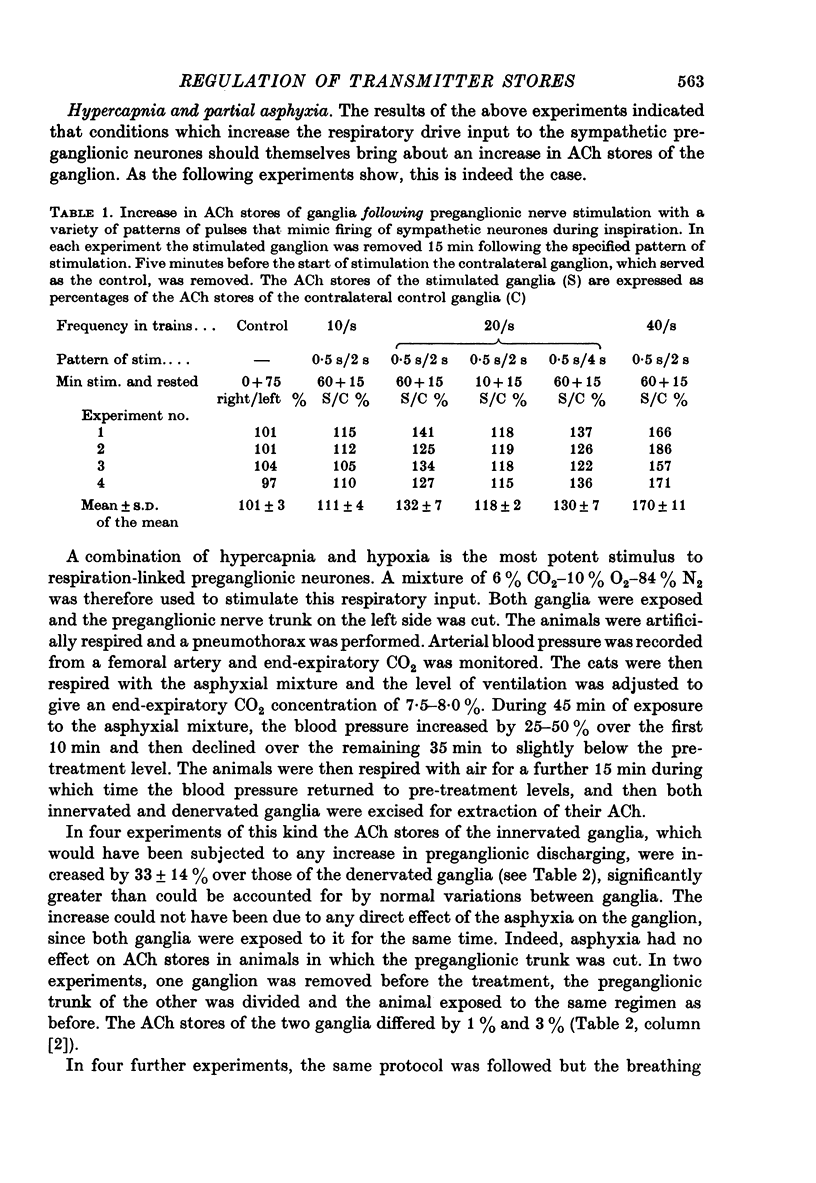

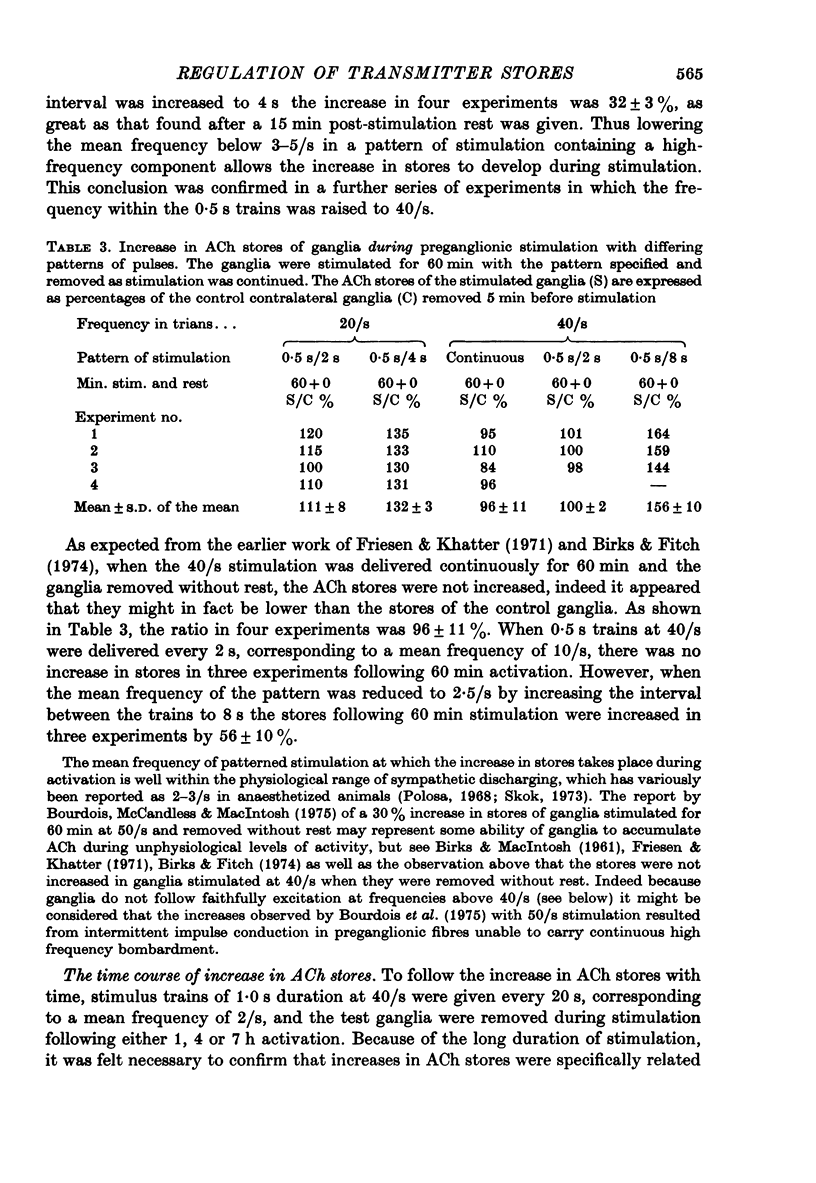
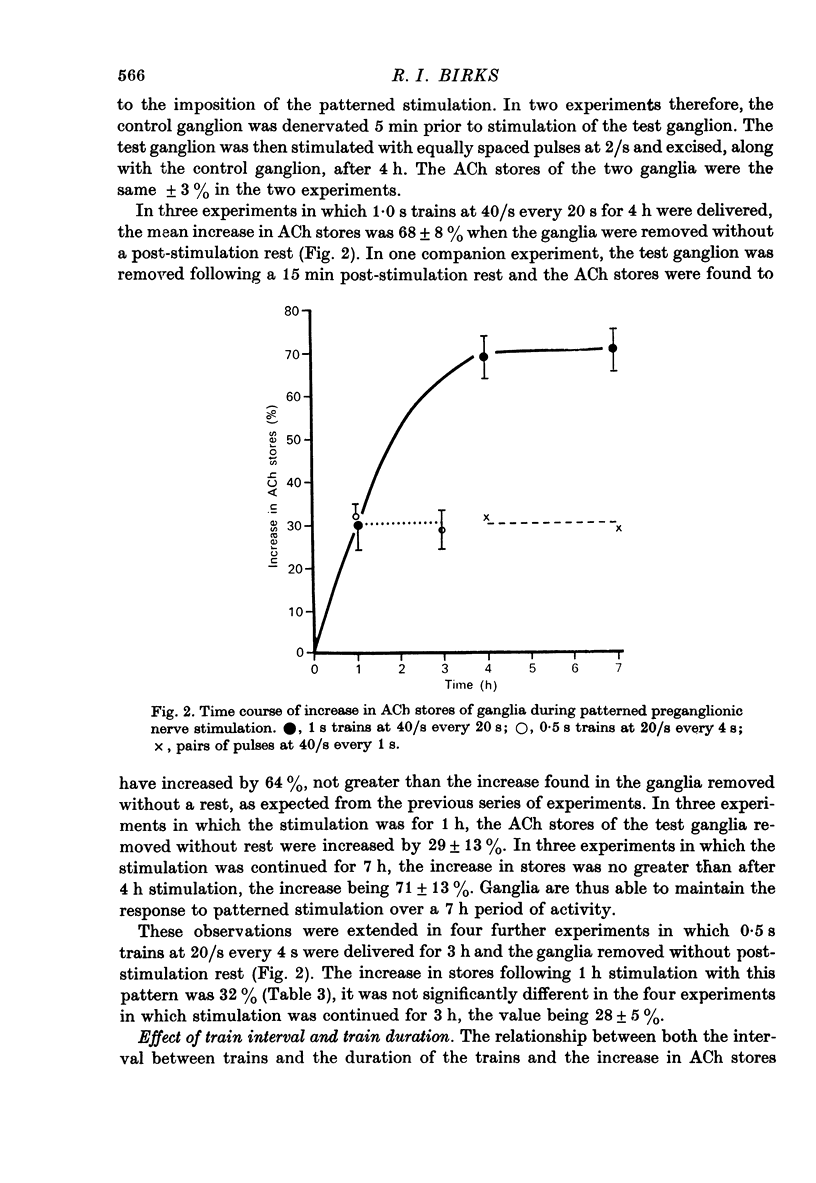





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birks R. I. A long-lasting potentiation of transmitter release related to an increase in transmitter stores in a sympathetic ganglion. J Physiol. 1977 Oct;271(3):847–862. doi: 10.1113/jphysiol.1977.sp012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Fitch J. G. Storage and release of acetylcholine in a sympathetic ganglion. J Physiol. 1974 Jul;240(1):125–134. doi: 10.1113/jphysiol.1974.sp010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I. The relationship of transmitter release and storage to fine structure in a sympathetic ganglion. J Neurocytol. 1974 Jun;3(2):133–160. doi: 10.1007/BF01098386. [DOI] [PubMed] [Google Scholar]
- Bourdois P. S., McCandless D. L., MacIntosh F. C. A prolonged after-effect of intense synaptic activity on acetylcholine in a sympathetic ganglion. Can J Physiol Pharmacol. 1975 Feb;53(1):155–165. doi: 10.1139/y75-022. [DOI] [PubMed] [Google Scholar]
- ECCLES R. M. Intracellular potentials recorded from a mammalian sympathetic ganglion. J Physiol. 1955 Dec 29;130(3):572–584. doi: 10.1113/jphysiol.1955.sp005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W. Synthesis of acetylcholine in sympathetic ganglia and cholinergic nerves. J Physiol. 1943 Mar 25;101(4):432–445. doi: 10.1113/jphysiol.1943.sp003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen A. J., Khatter J. C. The effect of preganglionic stimulation on the acetylcholine and choline content of a sympathetic ganglion. Can J Physiol Pharmacol. 1971 May;49(5):375–381. doi: 10.1139/y71-043. [DOI] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. After-potentials in mammalian non-myelinated nerve fibres. J Physiol. 1958 Dec 30;144(3):442–462. doi: 10.1113/jphysiol.1958.sp006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa C. Spontaneous activity of sympathetic preganglionic neurons. Can J Physiol Pharmacol. 1968 Nov;46(6):887–896. doi: 10.1139/y68-138. [DOI] [PubMed] [Google Scholar]
- Preiss G., Kirchner F., Polosa C. Patterning of sympathetic preganglionic neuron firing by the central respiratory drive. Brain Res. 1975 Apr 11;87(2-3):363–374. doi: 10.1016/0006-8993(75)90434-5. [DOI] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. J. Nitrous oxide--is it an adequate anaesthetic? J Physiol. 1973 May;231(1):20P–21P. [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. High affinity transport of choline into synaptosomes of rat brain. J Neurochem. 1973 Dec;21(6):1355–1374. doi: 10.1111/j.1471-4159.1973.tb06022.x. [DOI] [PubMed] [Google Scholar]


