Abstract
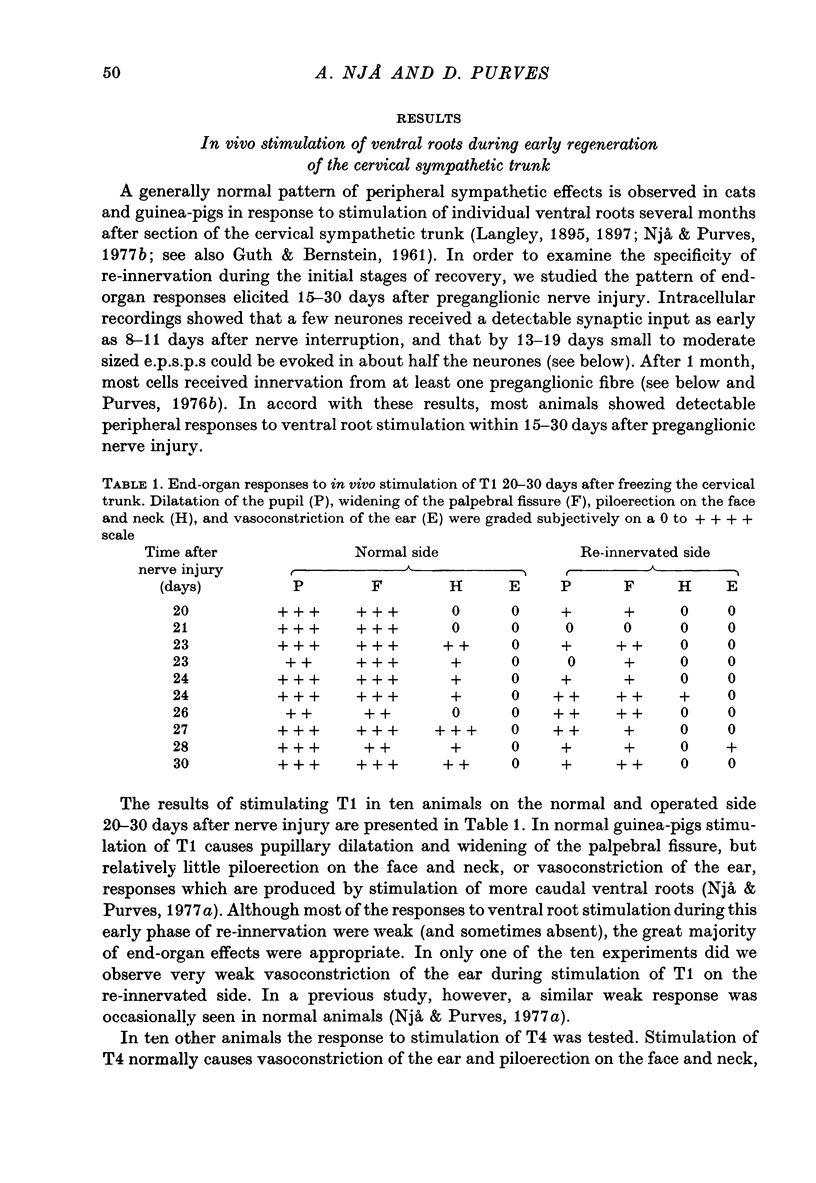
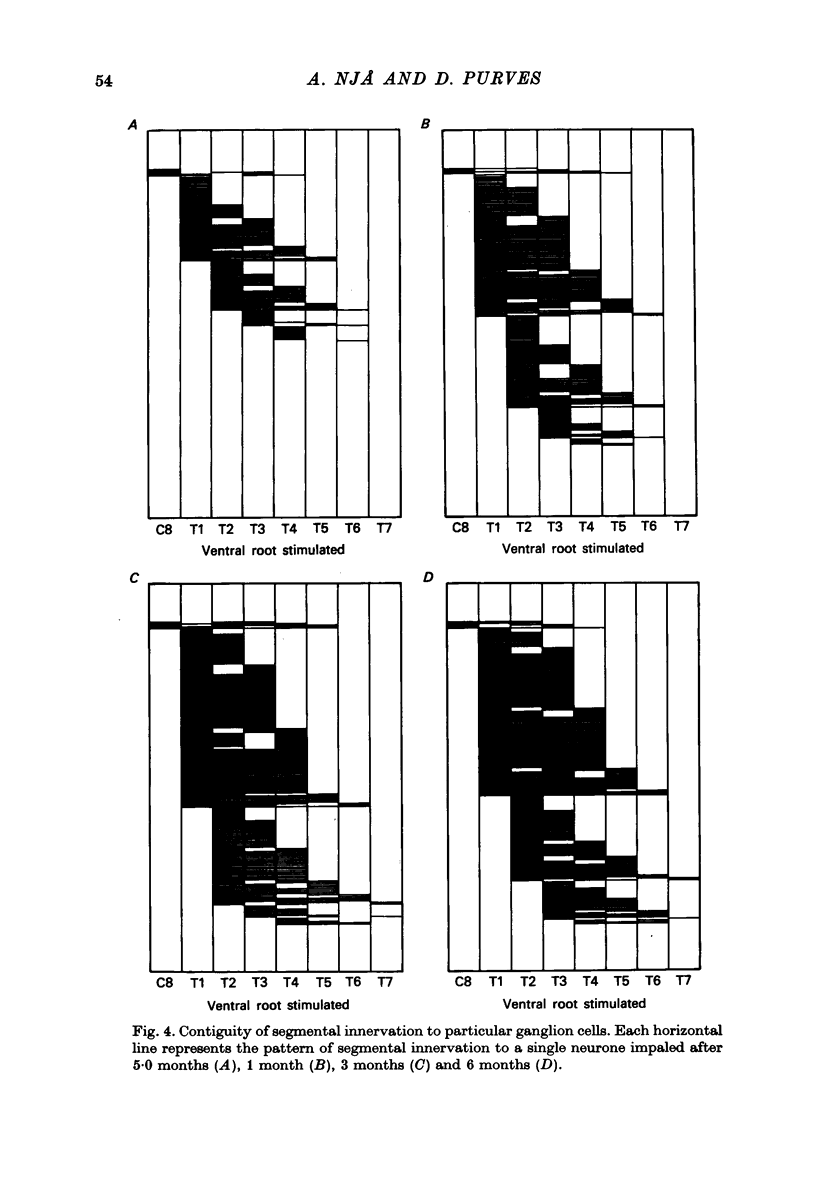
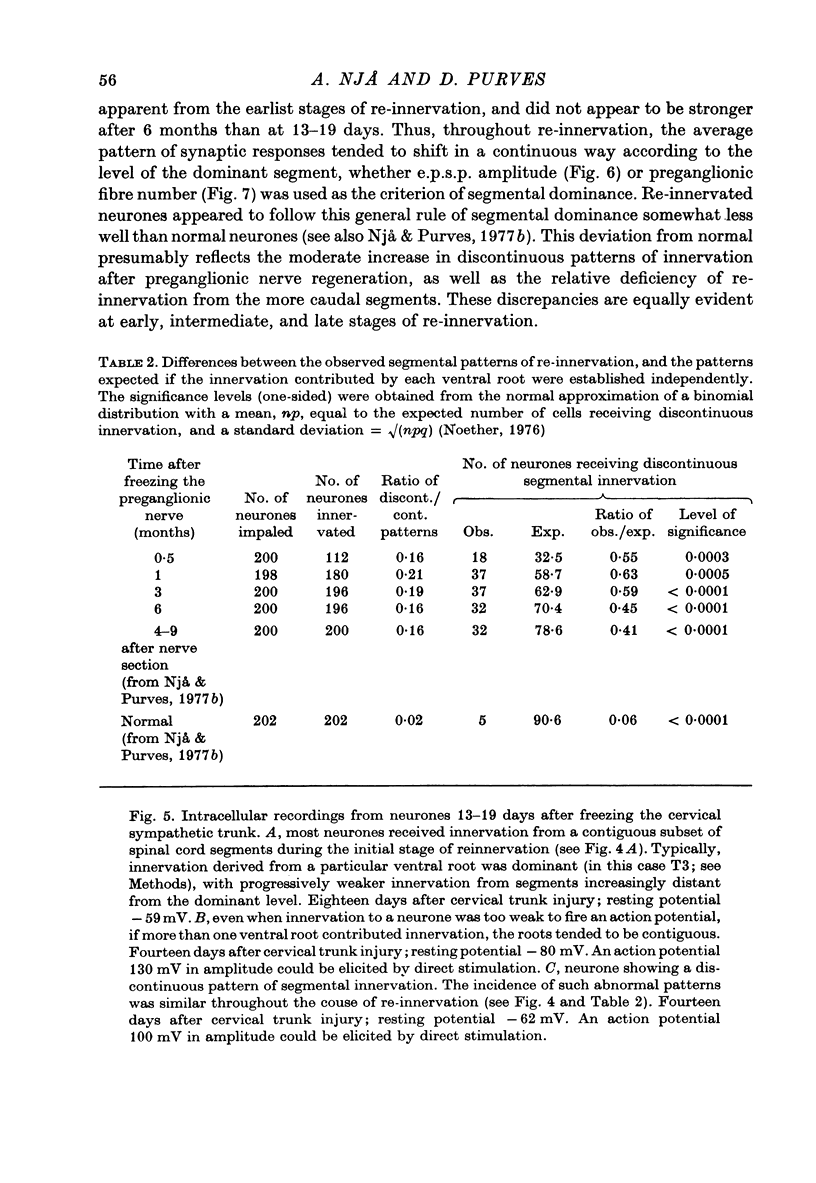
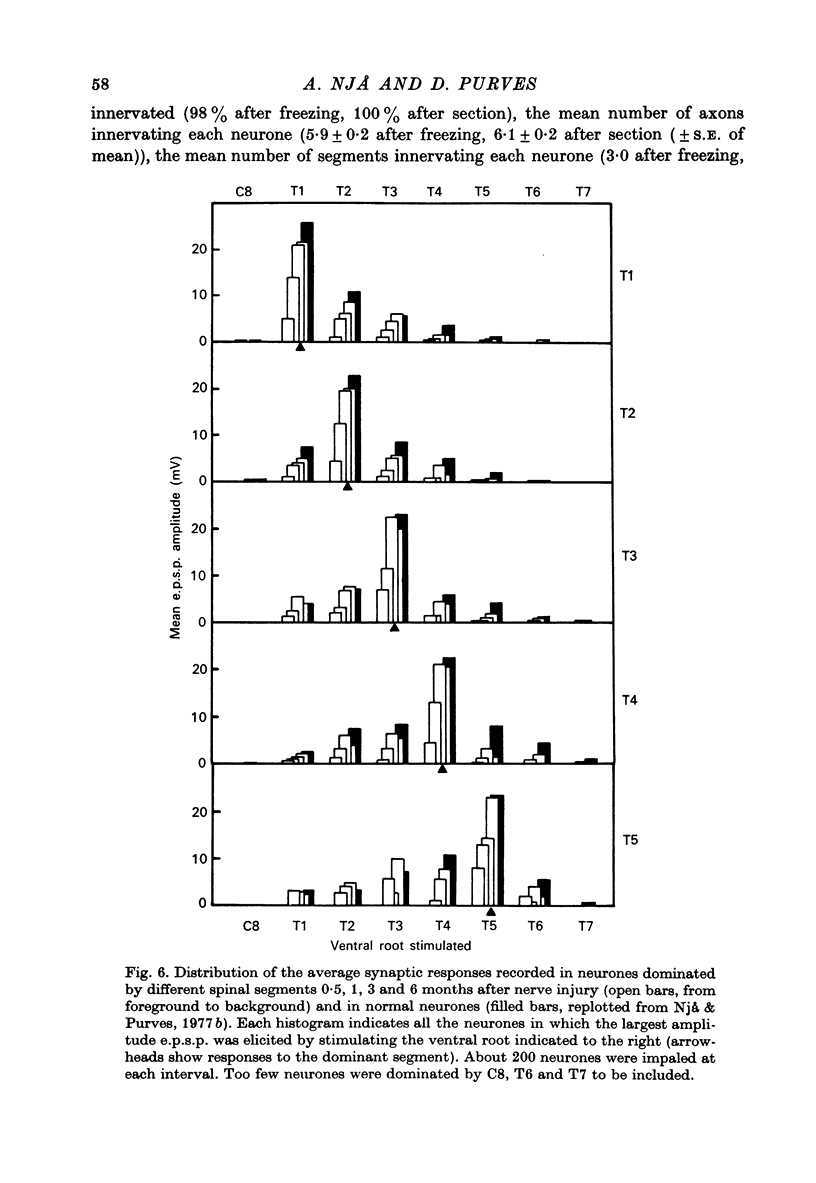
1. Largely appropriate synaptic connexions are formed with neurones in the superior cervical ganglion at long intervals after interruption of the preganglionic nerve. In the present study we have assessed the accuracy of connexions during the early stages of re-innervation by observing end-organ responses to ventral root stimulation in vivo, and by recording intracellularly from ganglion cells during ventral root stimulation in isolated preparations. 2. Appropriate, but weak, end-organ responses were elicited by stimulation of the first and fourth thoracic ventral roots (T1 and T4) 15--30 days after freezing the cervical sympathetic trunk. 3. Intracellular recordings from ganglion cells during stimulation of the ventral roots C8--T7 in vitro showed that synaptic contacts are first re-established 8--11 days after freezing the preganglionic nerve. The proportion of re-innervated cells, and the strength of innervation of individual neurones, increased rapidly for up to about 3 months after nerve injury, but showed little change thereafter. Innervation remained weaker than normal even after 6 months. 4. Patterns of segmental innervation recorded intracellularly during the early stages of regeneration were similar to, but more restricted than normal. Even 13--19 days after interruption of the preganglionic nerve, neurones re-innervated by more than one spinal cord segment tended to be innervated by a contiguous subset of the spinal segments which contribute innervation to the ganglion. The incidence of neurones receiving innervation from a discontinuous segmental subset was about the same at early and late stages or re-innervation. 5. Throughout the course of nerve regeneration, re-innervated neurones tended to receive dominant synaptic input from axons arising at a particular spinal level, as do normal cells, with adjacent segments contributing a synaptic influence that diminished as a function of distance from the dominant segment. 6. The results of these experiments argue against the initial formation of imprecise connexions with subsequent retention of appropriate contacts and a loss of inappropriate ones. Rather our findings suggest that the re-innervation of ganglion cells proceeds by a gradual accumulation of synaptic connexions which are, from the outset, appropriate.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATTARDI D. G., SPERRY R. W. Preferential selection of central pathways by regenerating optic fibers. Exp Neurol. 1963 Jan;7:46–64. doi: 10.1016/0014-4886(63)90093-1. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Motor neurone sprouting induced by prolonged tetrodotoxin block of nerve action potentials. Nature. 1977 Feb 3;265(5593):459–461. doi: 10.1038/265459a0. [DOI] [PubMed] [Google Scholar]
- GUTH L., BERNSTEIN J. J. Selectivity in the re-establishment of synapses in the superior cervical sympathetic ganglion of the cat. Exp Neurol. 1961 Jul;4:59–69. doi: 10.1016/0014-4886(61)90078-4. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Morris D. G. The development of functional innervation in the hind limb of the chick embryo. J Physiol. 1975 Jul;249(2):301–326. doi: 10.1113/jphysiol.1975.sp011017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Selective reinnervation of two cell populations in the adult pigeon ciliary ganglion. J Physiol. 1970 Nov;211(1):203–216. doi: 10.1113/jphysiol.1970.sp009275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. Note on Regeneration of Prae-Ganglionic Fibres of the Sympathetic. J Physiol. 1895 Jul 18;18(3):280–284. doi: 10.1113/jphysiol.1895.sp000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. On axon-reflexes in the pre-ganglionic fibres of the sympathetic system. J Physiol. 1900 Aug 29;25(5):364–398. doi: 10.1113/jphysiol.1900.sp000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. On the Regeneration of Pre-Ganglionic and of Post-Ganglionic Visceral Nerve Fibres. J Physiol. 1897 Nov 20;22(3):215–230. doi: 10.1113/jphysiol.1897.sp000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY J. G., THOMPSON J. W. The occurrence and function of collateral sprouting in the sympathetic nervous system of the cat. J Physiol. 1957 Jan 23;135(1):133–162. doi: 10.1113/jphysiol.1957.sp005700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M. R., Nelson V. H. Detachment of structurally intact nerve endings from chromatolytic neurones of rat superior cervical ganglion during the depression of synaptic transmission induced by post-ganglionic axotomy. J Physiol. 1975 Feb;245(1):91–135. doi: 10.1113/jphysiol.1975.sp010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nja A., Purves D. Re-innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Nov;272(3):633–651. doi: 10.1113/jphysiol.1977.sp012064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. Specific innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Jan;264(2):565–583. doi: 10.1113/jphysiol.1977.sp011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J Physiol. 1978 Apr;277:53–75. [PMC free article] [PubMed] [Google Scholar]
- Ostberg A. J., Raisman G., Field P. M., Iversen L. L., Zigmond R. E. A quantitative comparison of the formation of synapses in the rat superior cervical sympathetic ganglion by its own and by foreign nerve fibres. Brain Res. 1976 May 14;107(3):445–470. doi: 10.1016/0006-8993(76)90137-2. [DOI] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Casella C. Synaptically mediated potentials elicited by the stimulation of post-ganglionic trunks in the guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):55–67. doi: 10.1007/BF00587046. [DOI] [PubMed] [Google Scholar]
- Purves D. Competitive and non-competitive re-innervation of mammalian sympathetic neurones by native and foreign fibres. J Physiol. 1976 Oct;261(2):453–475. doi: 10.1113/jphysiol.1976.sp011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following colchicine application to post-ganglionic nerves. J Physiol. 1976 Jul;259(1):159–175. doi: 10.1113/jphysiol.1976.sp011459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G., Field P. M., Ostberg A. J., Iversen L. L., Zigmond R. E. A quantitative ultrastructural and biochemical analysis of the process of reinnervation of the superior cervical ganglion in the adult rat. Brain Res. 1974 May 10;71(1):1–16. doi: 10.1016/0006-8993(74)90187-5. [DOI] [PubMed] [Google Scholar]
- Van Essen D. C., Jansen J. K. The specificity of re-innervation by identified sensory and motor neurons in the leech. J Comp Neurol. 1977 Feb 15;171(4):433–454. doi: 10.1002/cne.901710402. [DOI] [PubMed] [Google Scholar]


