Abstract
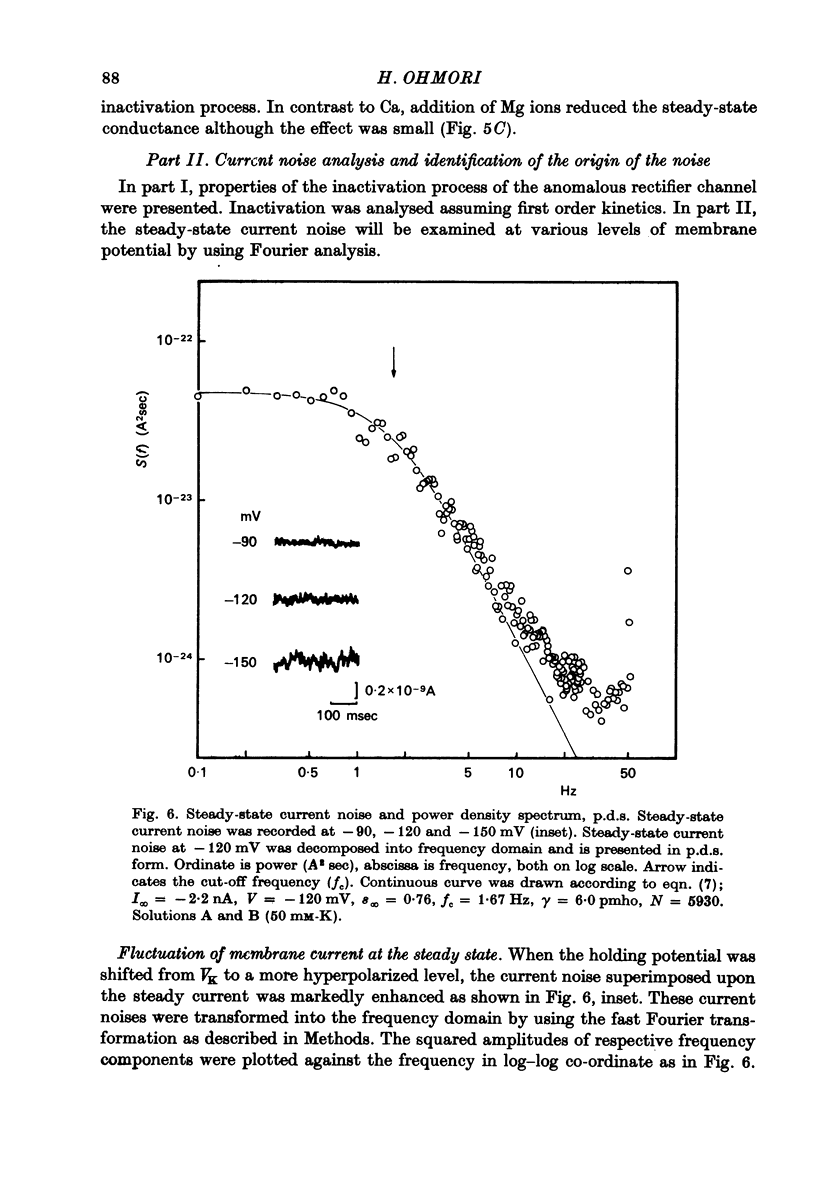
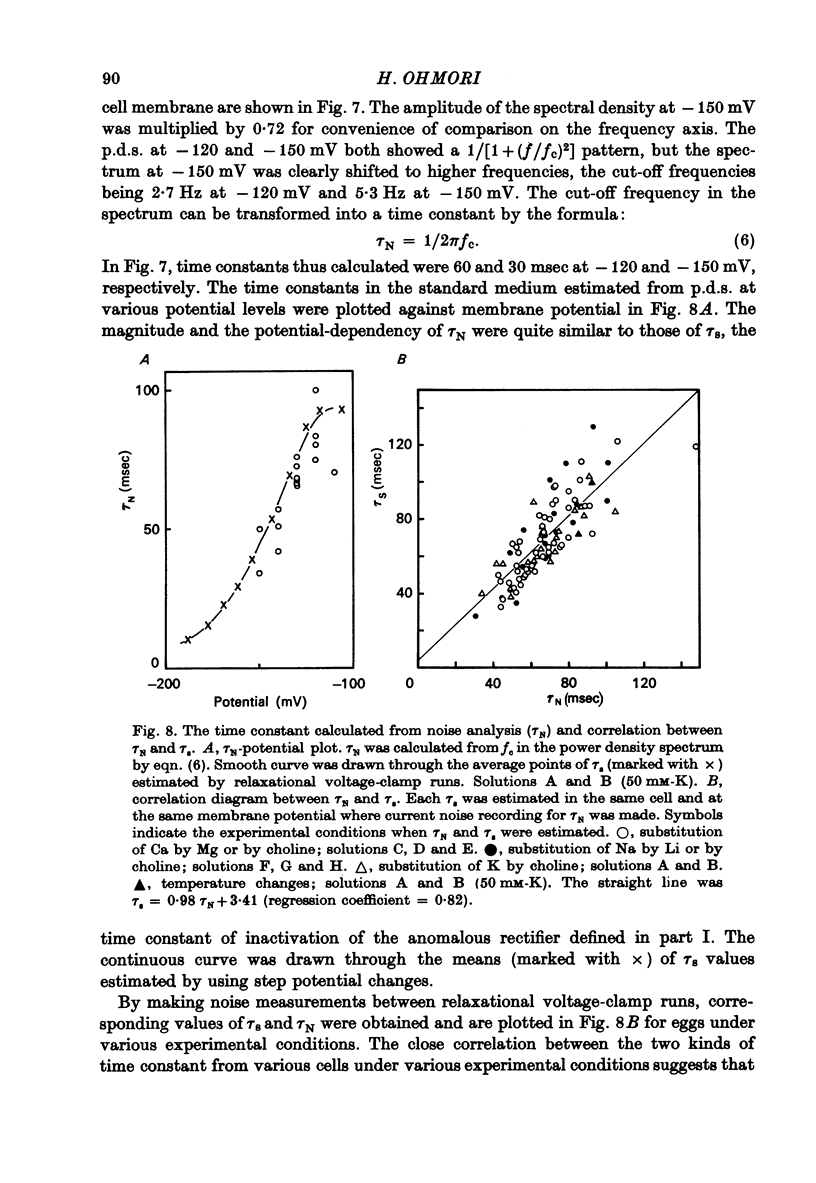
1. Inward K current through the anomalous rectifier in the tunicate egg (Halocynthis roretzi, Drashe) was studied under voltage clamp. The transient inward current in response to a step change of membrane potential was measured. The steady-state current fluctuations were analysed using the power density spectrum (p.d.s.). 2. The inward current showed time-dependent changes, which were described by a pair of the first order kinetic parameters, n and s for activation and inactivation, respectively. The steady-state channel open probability due to the activation process (n infinity) was assumed to be 1.0 for V more negative than about--100 mV, but that of the inactivation process (s infinity) and the time constant of inactivation (taus) were membrane potential dependent in the same potential range; both decreased with increasing hyperpolarization. 3. The inward currents in Na-free choline medium did not inactivate, but were decreased in size. In Na-free Li medium, inactivation was very small; the steady-state conductance was not affected significantly. 4. After exposure to high Ca media, an increase of the conductance was observed. This effect is probably caused by an increase of intracellular Ca due to Ca ions entering through the Na channels. Mg ions slightly decreased the conductance. 5. In the hyperpolarized membrane (-160 less than or equal to V less than or equal to -80mV), steady-state current noise was recorded and analysed using p.d.s. A p.d.s. of the 1/[1 + (f/fc)2] type as well a p.d.s. of the 1/f type was observed; f, frequency, fc, cut-off frequency. 6. fc was translated into time constant tauN (= 1/2pIfC) and compared with the time constant of inactivation, taus. There was a significant correlation betwen these values with a regression coefficient of 0.82. 7. Changing from 400 mM-Li abloshied inactivation and changed the p.d.s. from the 1/[1 + (f/fc)2] into the 1/f type. These results (paragraphs 5--7)suggest that the fluctuations in the steady-state currents originatte in the inactivation gatin kinetics of the an ofthe anomalous rectifier. 8. The number of anomalous rectifier channels and the unit channel conductance were estimated from the 1/[1 + (f/fc)2] type current noise according to the formula : (see text), where I infinity = gamma Nninfinity s infinity (V--VK), gamma the unit channel conductance, N the maximum number of channels that can be opened by a hyperpolarizing pulse per egg. The unit conductance was 6 pmho in standard artificial sea water and the channel density was 0.028/micrometer2. 9. The unit channel conductance (gamma) was dependent upon external K concentration, but the number ofchannels (N) was not. 10. The increase in chord conductance evoked by higher Ca concentrations was due to the increase of the channel number. By contrast, Mg ions seem to decrease the unit channel conductance slightly.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol. 1972 Aug;225(1):33–56. doi: 10.1113/jphysiol.1972.sp009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman C. Increase of sodium concentration near the inner surface of the nodal membrane. Pflugers Arch. 1970;317(4):287–302. doi: 10.1007/BF00586578. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J Gen Physiol. 1972 Nov;60(5):588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., De Felice L. J., Wanke E. Potassium and sodium ion current noise in the membrane of the squid giant axon. J Physiol. 1975 Jun;248(1):45–82. doi: 10.1113/jphysiol.1975.sp010962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. D. On the theory of ion transport across the nerve membrane. IV. Noise from the open-close kinetics of K + channels. Biophys J. 1972 Aug;12(8):948–959. doi: 10.1016/S0006-3495(72)86136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnberg G. Is potassium conductance of cardiac Purkinje fibres controlled by (Ca2+)? Nature. 1975 Jan 24;253(5489):273–274. doi: 10.1038/253273a0. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M., Rojas E. The binding of tetrodotoxin to nerve membranes. J Physiol. 1971 Feb;213(1):235–254. doi: 10.1113/jphysiol.1971.sp009379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. The temporal and steady-state relationships between activation of the sodium conductance and movement of the gating particles in the squid giant axon. J Physiol. 1976 Feb;255(1):157–189. doi: 10.1113/jphysiol.1976.sp011274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Lisiewicz A. Injections of calcium ions into spinal motoneurones. J Physiol. 1972 Sep;225(2):363–390. doi: 10.1113/jphysiol.1972.sp009945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson S. R., Meves H. The binding of tritiated tetrodotoxin to squid giant axons. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):349–352. doi: 10.1098/rstb.1975.0014. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K. Electrical excitability in the egg cell membrane of the tunicate. J Physiol. 1974 Apr;238(1):37–54. doi: 10.1113/jphysiol.1974.sp010509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K., Yoshii M. Analysis of non-linearity observed in the current-voltage relation of the tunicate embryo. J Physiol. 1974 Apr;238(1):55–77. doi: 10.1113/jphysiol.1974.sp010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Narahashi T., Shaw T. I. An upper limit to the number of sodium channels in nerve membrane? J Physiol. 1967 Jan;188(1):99–105. doi: 10.1113/jphysiol.1967.sp008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977 May;267(2):429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Membrane currents of the tunicate egg under the voltage-clamp condition. J Physiol. 1976 Jan;254(3):607–638. doi: 10.1113/jphysiol.1976.sp011249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Two components of the calcium current in the egg cell membrane of the tunicate. J Physiol. 1976 Feb;255(2):527–561. doi: 10.1113/jphysiol.1976.sp011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F. Inferences about membrane properties from electrical noise measurements. Biophys J. 1972 Aug;12(8):1028–1047. doi: 10.1016/S0006-3495(72)86141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verveen A. A., DeFelice L. J. Membrane noise. Prog Biophys Mol Biol. 1974;28:189–265. doi: 10.1016/0079-6107(74)90019-4. [DOI] [PubMed] [Google Scholar]


