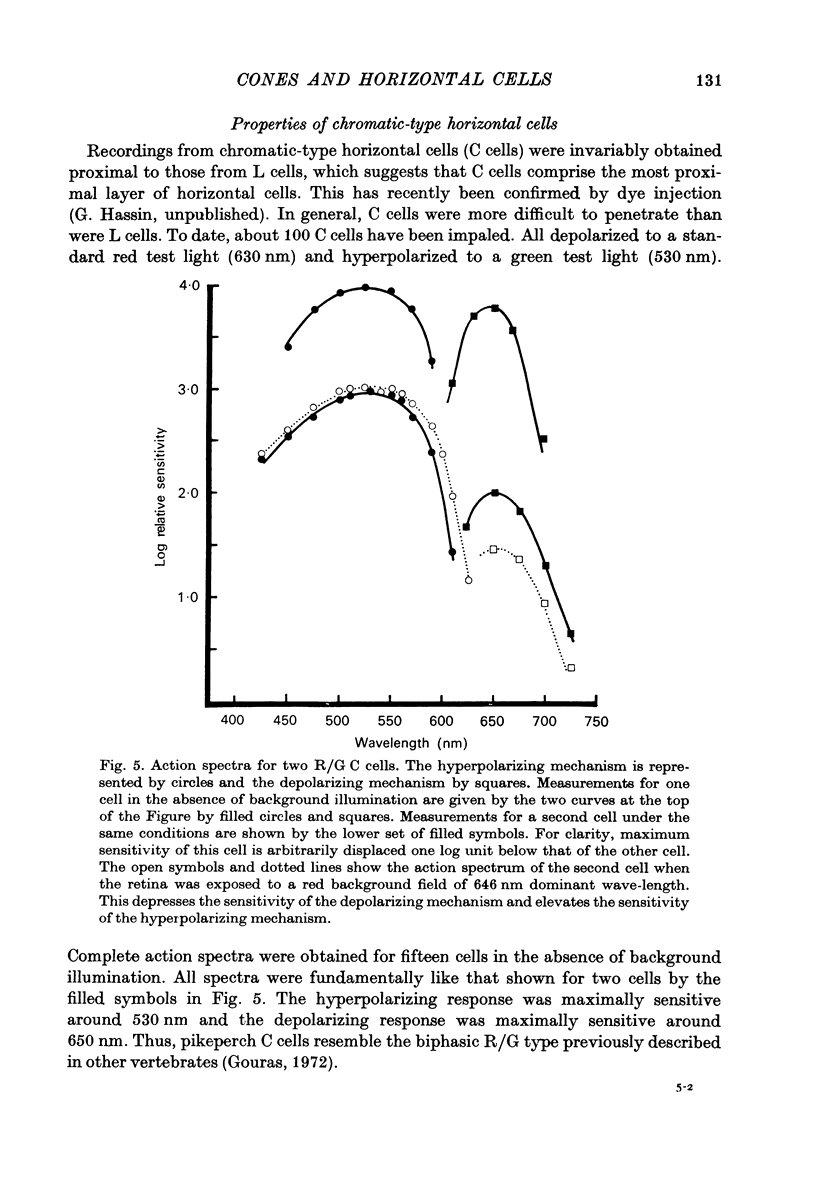
Abstract
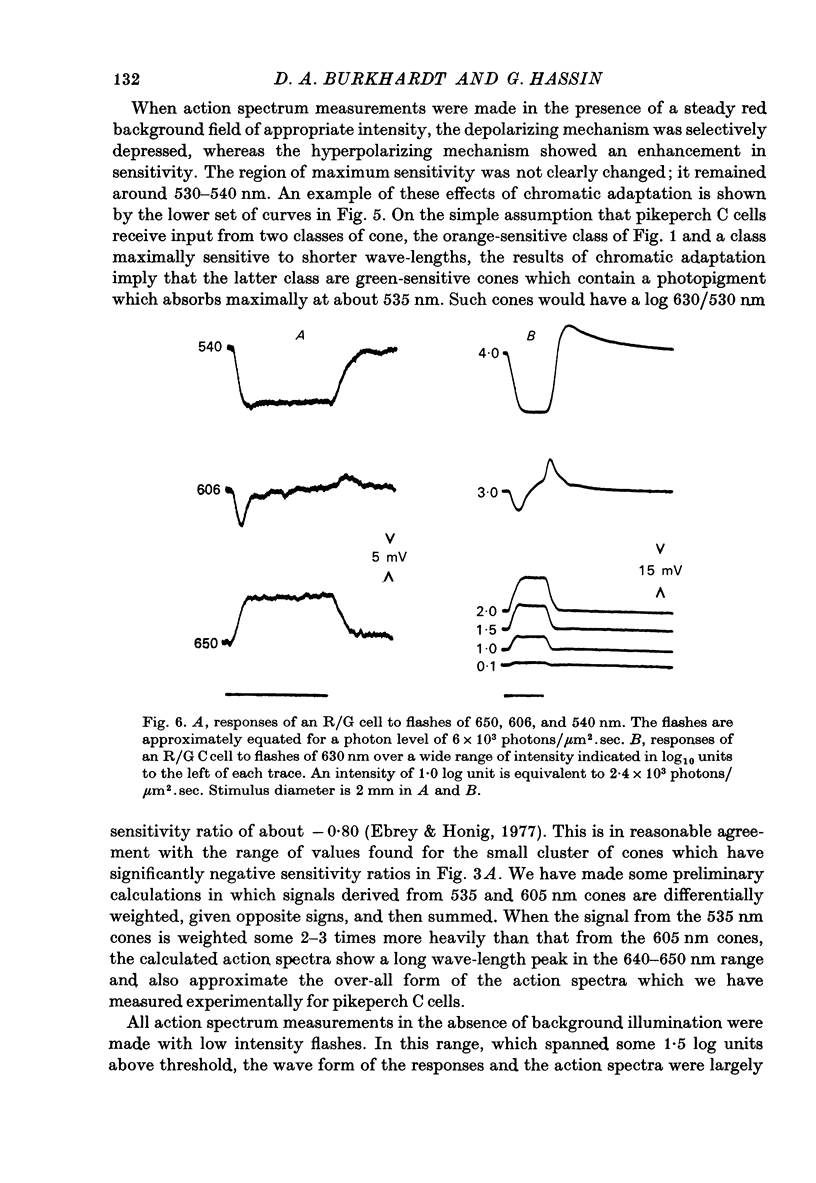
1. The spectral sensitivity and spatial organization of cones and horizontal cells have been analysed by intracellular recording in pikeperch retinas. 2. The vast majority of cone recordings were obtained from orange-sensitive cones. They have an action spectrum which peaks at about 605 nm. Recordings from several green-sensitive cones have also been obtained. 3. The results of action spectrum measurements and spectral screening tests indicate that the vast majority of luminosity-type horizontal cells receive predominant input from the orange-sensitive cones. 4. Chromatic-type horizontal cells were recorded at more proximal levels of the retina than luminosity-type cells and were the classic red-depolarizing, green hyperpolarizing (R/G) type. 5. The action spectra of the depolarizing and hyperpolarizing responses of chromatic horizontal cells peak at about 650 and 530 nm, respectively. When the depolarizing mechanism is selectively depressed by a red background field, the action spectrum of the hyperpolarizing mechanism shows an enhanced sensitivity, peaks at 530--540 nm, and may approximate the action spectrum of the green-sensitive cones. 6. Small red fields evoke depolarizing responses from chromatic-type horizontal cells but do not seem to significantly activate the depolarizing surround mechanism of cones. 7. These and other results suggest that the colour-opponent properties of the chromatic-type horizontal cells are not fundamentally dependent upon feed-back to cones but rather originate from antagonistic interactions generated in post-receptor networks.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
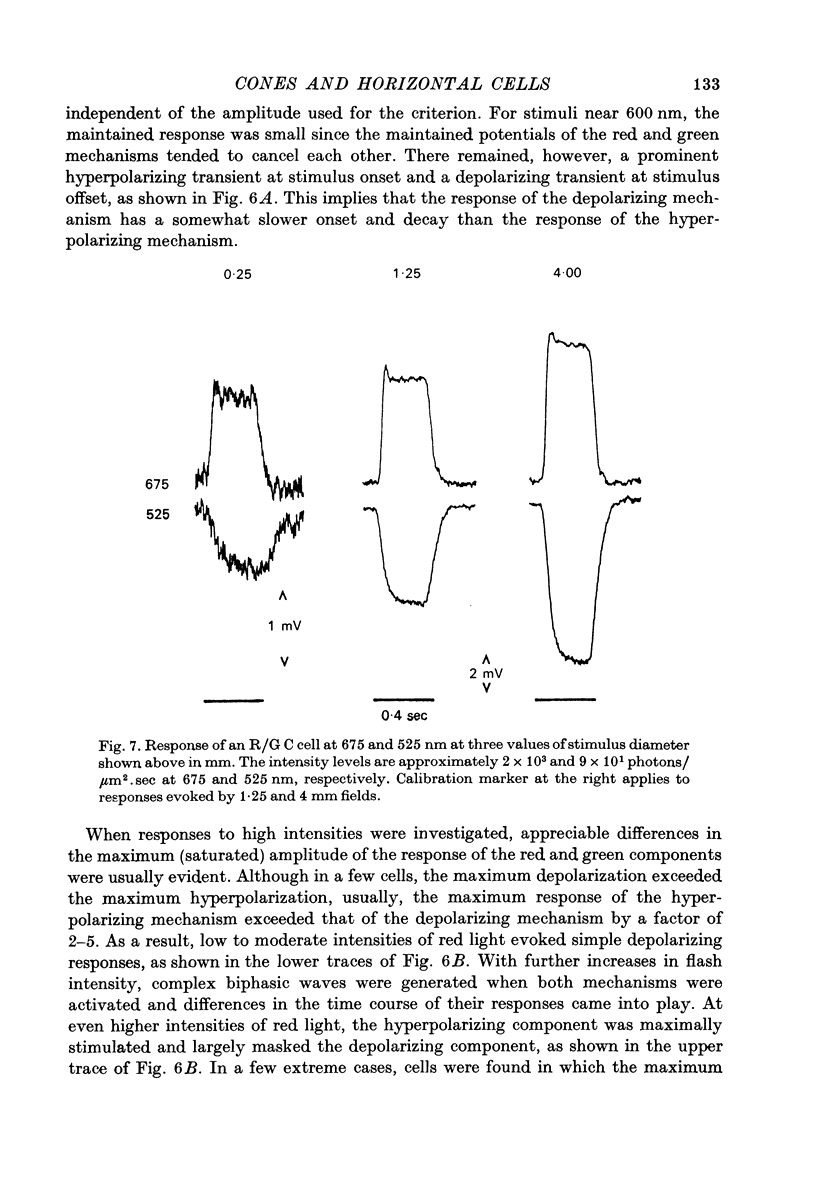
- Burkhardt D. A. Responses and receptive-field organization of cones in perch retinas. J Neurophysiol. 1977 Jan;40(1):53–62. doi: 10.1152/jn.1977.40.1.53. [DOI] [PubMed] [Google Scholar]
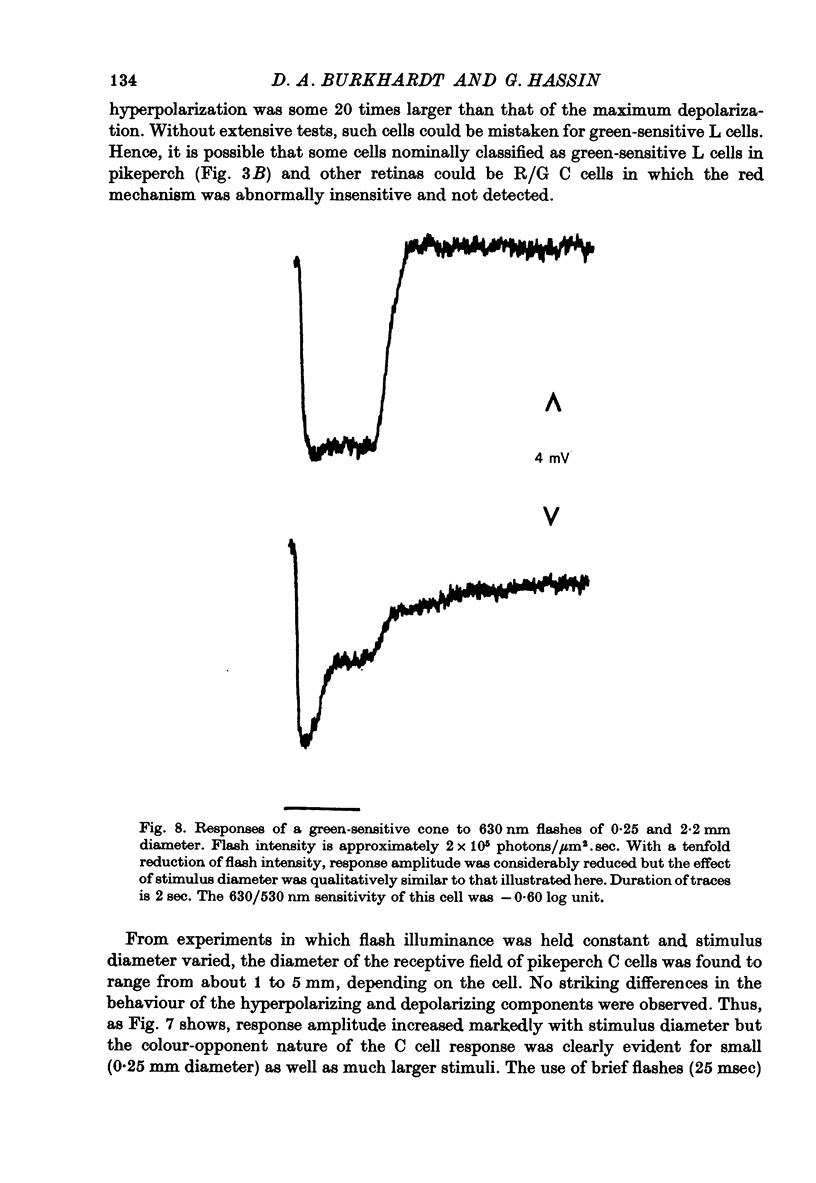
- Ebrey T. G., Honig B. New wavelength dependent visual pigment nomograms. Vision Res. 1977;17(1):147–151. doi: 10.1016/0042-6989(77)90213-9. [DOI] [PubMed] [Google Scholar]
- Fuortes M. G., Schwartz E. A., Simon E. J. Colour-dependence of cone responses in the turtle retina. J Physiol. 1973 Oct;234(1):199–216. doi: 10.1113/jphysiol.1973.sp010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuortes M. G., Simon E. J. Interactions leading to horizontal cell responses in the turtle retina. J Physiol. 1974 Jul;240(1):177–198. doi: 10.1113/jphysiol.1974.sp010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Kato A., Inokuchi M., Watanabe K. Re-examination of horizontal cells in the carp retina with procion yellow electrode. Vision Res. 1976 Jan;16(1):25–29. doi: 10.1016/0042-6989(76)90072-9. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Receptive field organization of bipolar and amacrine cells in the goldfish retina. J Physiol. 1973 Nov;235(1):133–153. doi: 10.1113/jphysiol.1973.sp010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer M., Millán E. Spectral analysis of L-type S-potentials and their relation to photopigment absorption in a fish (Eugerres plumieri) retina. Vision Res. 1970 Mar;10(3):237–251. doi: 10.1016/0042-6989(70)90129-x. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from luminosity units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):587–599. doi: 10.1113/jphysiol.1966.sp008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A comparison of electrical properties of neurons in Necturus retina. J Neurophysiol. 1973 May;36(3):519–535. doi: 10.1152/jn.1973.36.3.519. [DOI] [PubMed] [Google Scholar]
- Norton A. L., Spekreijse H., Wolbarsht M. L., Wagner H. G. Receptive field organization of the S-potential. Science. 1968 May 31;160(3831):1021–1022. doi: 10.1126/science.160.3831.1021. [DOI] [PubMed] [Google Scholar]
- Pinto L. H., Pak W. L. Light-induced changes in photoreceptor membrane resistance and potential in Gecko retinas. II. Preparations with active lateral interactions. J Gen Physiol. 1974 Jul;64(1):49–69. doi: 10.1085/jgp.64.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVAETICHIN G., MACNICHOL E. F., Jr Retinal mechanisms for chromatic and achromatic vision. Ann N Y Acad Sci. 1959 Nov 12;74(2):385–404. doi: 10.1111/j.1749-6632.1958.tb39560.x. [DOI] [PubMed] [Google Scholar]
- Stell W. K., Lightfood D. O., Wheeler T. G., Leeper H. F. Goldfish retina: functional polarization of cone horizontal cell dendrites and synapses. Science. 1975 Dec 5;190(4218):989–990. doi: 10.1126/science.1188380. [DOI] [PubMed] [Google Scholar]
- Tomita T., Kaneko A., Murakami M., Pautler E. L. Spectral response curves of single cones in the carp. Vision Res. 1967 Jul;7(7):519–531. doi: 10.1016/0042-6989(67)90061-2. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. A comparison of ganglion cell and S-potential response properties in carp retina. J Neurophysiol. 1967 May;30(3):546–561. doi: 10.1152/jn.1967.30.3.546. [DOI] [PubMed] [Google Scholar]
- Yazulla S. Cone input to bipolar cells in the turtle retina. Vision Res. 1976;16(7):737–744. doi: 10.1016/0042-6989(76)90184-x. [DOI] [PubMed] [Google Scholar]
- Yazulla S. Cone input to horizontal cells in the turtle retina. Vision Res. 1976;16(7):727–735. doi: 10.1016/0042-6989(76)90183-8. [DOI] [PubMed] [Google Scholar]


