Abstract
The pattern of meningococcal surface structure expression in different microenvironments following bloodstream invasion in vivo is not known. We used immunohistochemistry to determine the expression of capsule, type IV pili, and PorA by meningococci residing in the skin lesions of children with purpura fulminans. All the skin biopsy samples showed evidence of thrombosis and, frequently, a perivascular inflammatory cell infiltrate consisting of neutrophils (elastase positive) and monocytes/macrophages (CD68 positive). Modified Gram staining revealed 20 to over 100 gram-negative diplococci in each 4-μm-thick section, usually grouped into microcolonies. Immunoperoxidase staining demonstrated that the invading meningococci expressed PorA, capsule, and type IV pilin. Expression of these antigens was not restricted to any particular environment and was found in association with meningococci located in leukocytes, small blood vessels, and the dermal interstitium. Confocal laser scanning microscopy demonstrated coexpression of pilin and capsule by numerous microcolonies. However, there was some discordance in capsule and pilin expression within the microcolonies, suggesting phase variation. The strategy employed in this study will be helpful in investigating invasive bacterial diseases where antigenic and phase variation has a significant impact on virulence and on vaccine design.
Meningococcal infection, in the form of septicemia or meningitis, remains a significant cause of morbidity and mortality in industrialized countries and is associated with major epidemics in sub-Saharan Africa (8, 38, 42). Severe meningococcal sepsis is characterized by marked inflammatory cell activation, disseminated intravascular coagulation, and vascular compromise (13, 43). Purpura fulminans occurs frequently (in 10 to 20% of cases) and, in severe cases, results in thrombosis of large vessels with infarction of digits and limbs (19). Despite the apparent virulence of the organism, Neisseria meningitidis commonly colonizes the nasopharynx, only rarely causing overt disease (1). The meningococcus is exquisitely adapted to the process of nasopharyngeal adherence (30, 40, 56) and, following bloodstream invasion, has a striking ability to interact with vascular endothelium, polymorphonuclear neutrophils, and other inflammatory cells, enabling the organism to adapt to a variety of microenvironments (3, 9, 11, 12, 20, 26-28, 32, 33, 56, 59). It has been proposed that these pathogen-host cell interactions occur through a multistep process of adherence and invasion, mediated by the binding of meningococcal ligands to host cell receptors normally utilized for host cell-to-host cell targeting (34, 60). The meningococcus has developed a remarkable ability to alter surface-exposed molecules under a variety of selective environmental pressures (44). A fuller understanding of the expression pattern of key bacterial surface molecules following bloodstream invasion is therefore vital to our understanding of meningococcal pathogenesis. Such data will also provide important insight into the availability of meningococcal antigens as vaccine antigens in vivo. The major meningococcal outer membrane protein PorA, for example, forms the basis of the serogroup B vaccines that have been most extensively investigated in human clinical trials (4, 36, 46, 53). However, PorA expression can be varied by multiple mechanisms that could potentially limit vaccine efficacy (53).
In recent years, in vitro models have significantly advanced our understanding of meningococcus-host cell interactions. Initial attachment of the meningococcus is thought to be mediated by type IV pili via the host cell receptor CD46, a complement regulatory protein (25, 29, 37, 55, 59). Pili are ubiquitous in clinical isolates from patients with meningococcal sepsis and initiate the cytoskeletal rearrangements required for invasion. Prior to invasion, piliation is thought to be lost and secondary, tight association is conferred by a number of putative meningococcal adhesins, which include the Opa and Opc proteins, lipooligosaccharide, and the porins (34, 56, 58, 59, 61). The host cell receptors for these bacterial adhesins have not been fully defined and appear to vary between cell types but include carcinoembryonic antigen cell adhesion molecules, the integrin αvβ3, and heparan sulfate proteoglycans. This process of adhesion and subsequent close association occurs most efficiently when Opa is expressed by unencapsulated meningococci. However, the polysaccharide capsule is thought to confer resistance to nonspecific immune responses including complement activation and, in the case of serogroup B N. meningitidis, evasion of adaptive immune surveillance through similarities between capsule and NCAM-1 on neuronal tissue. Whether invasive meningococcal strains vary capsule expression to facilitate Opa-mediated host cell interactions remains to be determined.
The proposed paradigm of meningococcus-host cell interactions, particularly following bloodstream invasion, is limited by the fact that it has largely been based on observations made from laboratory models. For example, human umbilical vein endothelial cells are a widely accepted model of the postcapillary venule (16), but such cell culture systems do not reflect the complex microenvironment occupied by the meningococcus during disease. There is no good animal model of meningococcal sepsis, and there is only a limited amount of data describing variations in meningococcal gene expression at different anatomic sites in the human host (35, 52, 62, 63). Therefore, the pattern of meningococcal surface antigens expressed during interactions associated with the human microvasculature during disease is currently unknown.
In purpura fulminans, the typical skin rash is characterized by dermal microvascular thrombosis and perivascular hemorrhage (19). Within these lesions, multiple meningococci have been reported to be associated with endothelial cells, leukocytes, and thrombi and in the extravasated material from the damaged vessels (23, 47). In this study, we have investigated the hypothesis that, following bloodstream invasion, there is variation in the pattern of meningococcal surface antigen expression in different cellular microenvironments in vivo. We used a unique collection of skin biopsy samples from children with severe meningococcal disease to establish the expression pattern of capsule, PorA, and type IV pili by N. meningitidis in different locations within the skin. (Portions of this work were presented at the 12th International Pathogenic Neisseria Conference, Galveston, Texas, 2000.)
MATERIALS AND METHODS
Subjects and skin biopsies.
Three-millimeter-diameter punch biopsy samples were collected from the edges of purpuric lesions from 83 consecutive patients with meningococcal sepsis (median age, 41 months [range, 1 to 185]; median Glasgow Meningococcal Prognostic Score, 11 of 15 [range 6 to 15]) (14, 51). The collection of the biopsy samples and the research described complied with all relevant guidelines and institutional practices (St. Mary's Local Research Ethics Committee, EC 3396). Meningococcal disease was diagnosed by clinical criteria and confirmed by culture, antigen detection, and/or PCR (Public Health Laboratory Service Meningococcal Reference Unit, Manchester, United Kingdom). All biopsy samples were taken less than 24 h from the time the first dose of parenteral antibiotics was administered and immediately fixed in formol saline or collected in optimal cutting temperature medium and then snap-frozen in liquid nitrogen. For this study, 13 biopsies were selected at random: 5 formalin-fixed paraffin-embedded biopsy samples for which the characteristics of the infecting strain were also available (N. meningitidis B:NT:P1.15, B:15:P1.16, B:NT:P1.9, and B:NT:P1.4 and N. meningitidis C:2.2a:P1.2, 5) and 8 frozen biopsy samples for which the characteristics of the infecting strain were also available (N. meningitidis B serogroups B:NT:P1.10, B:4:P1.4, and B:NT:P1.5; 3 N. meningitidis B serogroups, serosubtypes unknown; and N. meningitidis C serogroups C:2a:P1.4 and C:2a:P1.10). Control skin was taken from children undergoing routine surgical procedures after informed parental consent.
Antimeningococcal antibodies.
Mouse monoclonal anti-capsule C and -capsule B and anti-PorA antibodies were obtained from the National Institute for Biological Standards and Control (NIBSC), South Mimms, Herts, United Kingdom. Mouse monoclonal antipilin antibody (SM1) was a kind gift from M. Virji.
Assessment of antimeningococcal antibodies.
The utility of the antimeningococcal monoclonal antibodies in different staining settings was assessed by indirect immunofluorescence and immunoblotting and in a human conjunctival epithelial cell infection model. This was undertaken by using the invasive isolates corresponding to the skin biopsy samples used for the immunohistochemistry experiments (N. meningitidis B serogroups B:NT:P1.15, B:15:P1.16, B:NT:P1.9, and B:NT:P1.4 and C serogroup C:2.2a:P1.2, 5). Stationary-phase meningococci grown overnight on gonococcal agar (Difco, Detroit, Mich.) supplemented with 1% Vitox (Oxoid, Basingstoke, Hampshire, England) were used throughout. Screening by immunofluorescence was conducted by staining suspensions of the invasive meningococcal strains adherent to poly-l-lysine-coated multispot slides (Hendley, Essex, United Kingdom) following blocking with 10% fetal calf serum (FCS). Specific binding was then detected with anti-mouse immunoglobulin conjugated to fluorescein isothiocyanate or tetramethyl rhodamine isocyanate (Dako) (54). Immunoblotting was performed with whole-cell lysates of the meningococcal strains boiled in 4% sodium dodecyl sulfate-20% glycerol-10% 2-mercaptoethanol-0.04% bromphenol blue-0.125 M Tris-HCl for 5 min and then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a 10% acrylamide running gel (24). Antigens were transferred onto a nitrocellulose blotting membrane (Gelman Sciences, Longmont, Colo.) with a semidry blotter (GmbH; Biometra biomedizinische Analytik). The membranes were then blocked with 4% bovine serum albumin and stained with the primary antibodies. Specific antibody binding was detected with anti-mouse immunoglobulin horseradish peroxidase (Dako) and visualized with diaminobenzidine. For the infection studies, human Chang conjunctiva epithelial cells (clone 1-5c-4; European Collection of Cell Cultures) were grown to confluence in 75-cm2 flasks (Nunclon, Roskilde, Denmark) (45). These cells were then inoculated with suspensions of the invasive meningococcal strains (final concentration, 107 cells/ml) and incubated overnight. After washing, the cells were detached with nonenzymatic cell dissociation solution (Sigma-Aldrich, Gillingham, United Kingdom). The cells were then divided into 1-ml aliquots and centrifuged at 11,000 × g for 5 min. The supernatant was decanted, and 100 μl of 10% molten agar in 10% formol saline was added to each aliquot. Next, this mixture was centrifuged for 15 s at 11,000 × g and then left to set overnight at 4°C. The agar pellets were scooped out and paraffin embedded in a biopsy cassette (Shandon, Pittsburgh, Pa.). Four-micrometer-thick sections were cut and mounted onto electrostatically charged microscope slides (BDH, Lutterworth, United Kingdom) and then immunostained as described below.
Immunohistochemistry.
Hematoxylin and eosin staining was undertaken to determine the pathological characteristics of each of the five formalin-fixed paraffin-embedded biopsy samples. Resident meningococci were identified in consecutive sections by modified Gram stain (6), and their surface characteristics were determined by immunostaining (21) with mouse monoclonal antibodies against capsule (serogroups B and C), pilin (SM1), and PorA (the appropriate antibody based on the serotype of the infecting strain). Blood vessels, monocytes/macrophages, and neutrophils were identified with CD31 (mouse anti-human monoclonal antibody JC/70A, isotype immunoglobulin G1 [IgG1]; Dako), CD68 (mouse anti-human monoclonal antibody EBM11, isotype IgG1; Dako), and neutrophil elastase (mouse anti-human monoclonal antibody NP57, isotype IgG1; Dako), respectively. Briefly, sections were incubated in 0.3% aqueous hydrogen peroxide to quench endogenous peroxidase activity and blocked with normal mouse or rabbit serum before incubation with the monoclonal antibodies for 1 h at room temperature. Labeling was detected with an immunoperoxidase kit (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, Calif.), and sections were counterstained in hematoxylin. Antigen retrieval treatment of the sections was required for optimal staining with all of the meningococcal monoclonal antibodies and the anti-CD31 and -CD68 antibodies (21). This consisted of either enzymatic digestion at 37°C with trypsin (1 mg/100 ml from porcine pancreas; ICN Biomedicals) for 20 min (antibodies to serogroup B, pilin, and PorA) or microwave antigen retrieval in citrate buffer (HDS05, pH 6.0; SD Supplies, Aylesbury, United Kingdom) diluted in 100% glycerol for the anti-capsule serogroup C antibody. Antigen retrieval was required because formalin fixation causes aldehyde bonds to form between proteins, which, while preserving the biopsy sample, may also generate a netlike effect around the tissue, masking some of the antigen epitopes (21). Proteinase digestion and heat treatment were employed to break these bonds and so revealed the antigens under study. Negative controls included the omission of the primary and secondary antibodies and staining of control skin sections. The relationship between meningococcal antigen expression and the location of the bacteria within the skin (i.e., in the interstitium, associated with blood vessels or leukocytes) was determined by semiquantitative analysis. For each biopsy sample, the resident meningococci were initially identified by Gram stain. Sections were then scored for N. meningitidis pilin, capsule, and PorA expression as follows: 1+, 1 to 20 meningococci were seen; 2+, 20 to 40 meningococci were seen; 3+, 40 to 60 meningococci were seen; 4+, over 60 meningococci were seen. Accuracy of section scoring was monitored by random double-blind assessment. The sections were re-reviewed blind and independently by two of us to ensure consistency and the accuracy of the interpretation. Photographs were taken with a Nikon Eclipse E400 microscope, a Nikon HIII photomicrograph, and a Nikon FDX-35 camera. Natural light was enhanced for photography with a color-balancing filter (NCB11; Nikon).
Confocal laser scanning microscopy.
To further investigate the cellular distribution of the resident meningococci and perform double staining, biotin-coupled capsule antibodies were generated. The unlabeled monoclonal antibody was incubated for 2 h at room temperature in a 1:1 mixture of 2 mg of antibody/ml and 50 mg of Biotin (Long Arm) N-hydroxysuccinimide (Vector Laboratories)/ml. The biotinylated antibody was then dialyzed in 2 liters of phosphate-buffered saline (PBS) overnight at 4°C with stirring by using a 10,000-kDa-molecular mass cutoff 0.1- to 0.5-ml sample volume dialysis cassette (Slide-A-Lyzer; Pierce, Rockford, Ill.) according to the manufacturer's instructions. Biotinylation of antibodies was then confirmed by enzyme-linked immunosorbent assay and immunofluorescence staining.
Thick sections (7 μm) were obtained from eight frozen skin biopsy samples (see above) and immunostained by modification of the method described by Richter-Dahlfors et al. (41). Following initial permeabilization with 0.5% saponin, the sections were briefly fixed with fixing buffer (0.2% glucose and 2% formaldehyde in PBS) and then blocked with 10% FCS-PBS for 30 min. Autofluorescence was quenched with incubation in 0.1% sodium borohydride-PBS, after which the sections were washed in 0.01% Tween 20-PBS. Nonspecific binding of endogenous biotin-streptavidin was blocked for 30 min with a blocking kit obtained from Vector Laboratories. The sections were then incubated for 1 h at 37°C with the biotin-coupled anticapsule meningococcal antibody (diluted 50 μg/ml in PBS containing 0.2% saponin and 10% FCS), which was then detected by incubation for 1 h at room temperature with a streptavidin-linked fluorochrome (streptavidin-conjugated Alexa Fluor 488 [Molecular Probes], diluted 10 μg/ml in PBS containing 1% FCS). After the sections were washed in 0.01% Tween 20-PBS, they were incubated for a further hour at 37°C with the antipilin or anti-PorA meningococcal antibodies, both of which were subsequently detected with a fluorescent secondary antibody (Alexa Fluor 594 rabbit anti-mouse antibody [Molecular Probes], diluted 10 μg/ml in PBS containing 1% FCS). The signal was then amplified with another secondary antibody (Alexa Fluor 594 goat anti-rabbit antibody [Molecular Probes], diluted 10 μg/ml in PBS containing 1% FCS). Fluorescence was preserved using a commercial mounting medium (Prolong Antifade kit; Molecular Probes). Sections were then viewed by using Leica TCS-NT and Zeiss LSM 510 confocal laser scanning compound microscopes (63× lens objective) with settings to optimize resolution. Optical sections were taken at 1-μm intervals.
RESULTS
Thrombosis and inflammation in the skin of children with purpura fulminans.
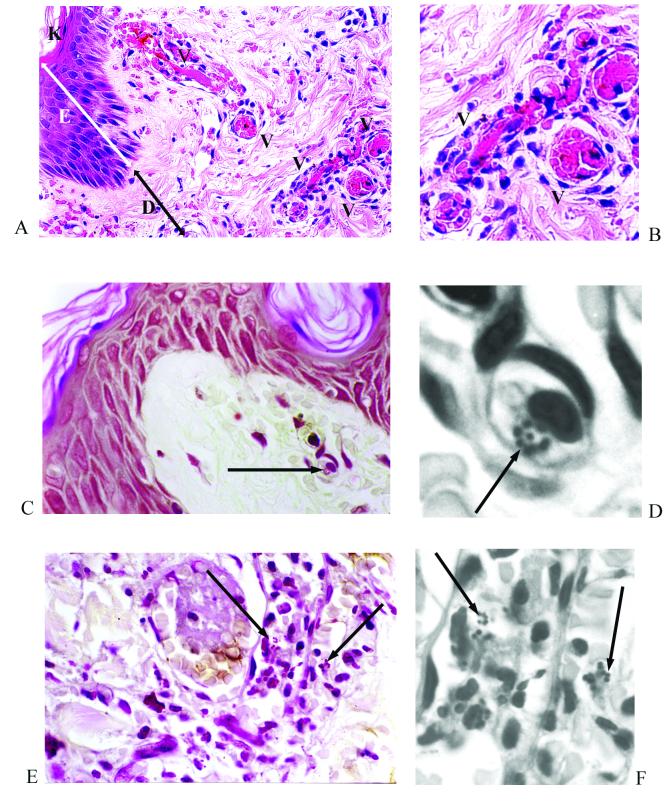
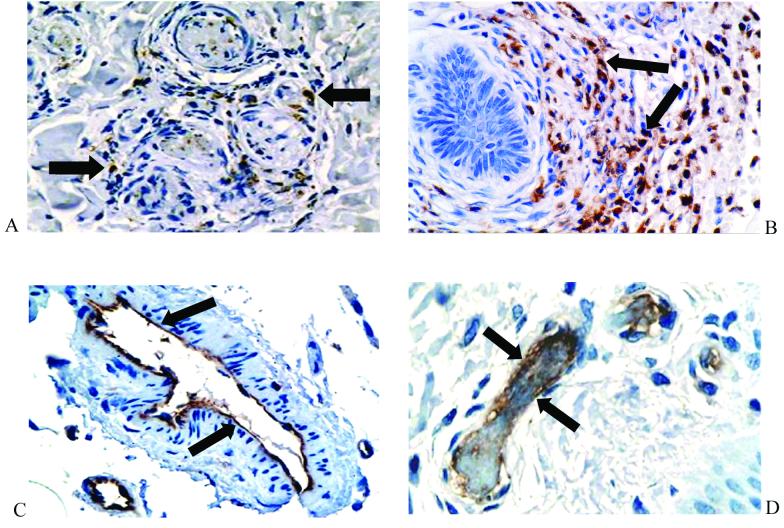
Histological review of biopsy samples taken from the edges of characteristic purpuric skin lesions revealed that, in all cases, the general tissue structure was well preserved. In all the biopsy samples, there was evidence of thrombosis and, frequently, a perivascular inflammatory cell infiltrate (Fig. 1A and B). Immunoperoxidase staining revealed that this infiltrate consisted of neutrophils (neutrophil elastase positive) and monocytes/macrophages (CD68 positive) (Fig. 2A and B). The endothelia of small blood vessels throughout the tissue stained positive for CD31 (Fig. 2C and D). Transmission electron microscopy has been previously used to show that, while occasional vessels with or without thrombosis showed endothelial loss with characteristic lack of organelles, this was not widespread (14).
FIG. 1.
Skin biopsy specimen from a patient with meningococcal septicemia. (A and B) An area of endothelial swelling, acute inflammation with a perivascular infiltrate, and thrombosis. Panel A shows hemotoxylin and eosin staining at a magnification of ×400; panel B was electronically enlarged. (C through F) Gram-stained sections reveal meningococci associated with a leukocyte (panel C, magnification at ×400; panel D, electronically enlarged) and within blood vessels (panel E, magnification at ×400; panel F, electronically enlarged). Figures were processed with Adobe Photoshop and illustrator software. K, keratin layer; E, epidermis; D, dermis; V, blood vessel. Arrows indicate Gram-stained meningococci.
FIG. 2.
Immunohistochemical staining of inflammatory cells and the vascular endothelium in skin biopsy samples from patients with meningococcal disease. The inflammatory infiltrate consisted of a mixture of CD68-positive macrophages (A) (magnification, ×400) and neutrophil elastase-positive polymorphonuclear cells (B) (magnification, ×400). Multiple CD31-positive blood vessels were seen throughout the biopsy samples (C and D) (magnification, ×400 and 600, respectively). Arrows indicate positive immunoperoxidase staining (brown) with the appropriate specific mouse monoclonal antibody (nuclei were counterstained with hematoxylin).
N. meningitidis in the skin lesions of patients with purpura fulminans.
Modified Gram staining has previously revealed microcolonies of meningococci in 23 of 25 biopsy samples that we have surveyed (unpublished results). In the five biopsy specimens analyzed in this study by Gram staining and immunoperoxidase, we observed from 20 to over 100 clearly visible gram-negative diplococci in each section, usually as microcolonies (Fig. 1). These were localized to leukocytes, small blood vessels, and the dermal interstitium. There was no relationship between the severity of the clinical disease (as measured by Glasgow Meningococcal Prognostic Score) or the histological changes and the number of meningococci identified.
Evaluation of antimeningococcal antibodies in vitro.
By using the five invasive meningococcal isolates associated with the five formalin-fixed paraffin-embedded biopsy samples, assessment of mouse monoclonal antibodies to N. meningitidis capsule, pilin, and PorA revealed strong immunofluorescence staining of immobilized meningococci and clear immunoperoxidase staining of the strains in the Chang cell adherence model (results not shown). PorA staining was serosubtype specific with no observed cross-reactivity. Reactivity to pilin (a single band) and capsule (a broad smear [15]) was observed by Western blot analysis (results not shown). PorA was not detectable by Western blot analysis with the anti-PorA monoclonal antibody, suggesting that the antibody binds to an epitope denatured during processing (48).
In vivo expression of capsule, pilin, and PorA in different microenvironments within the skin.
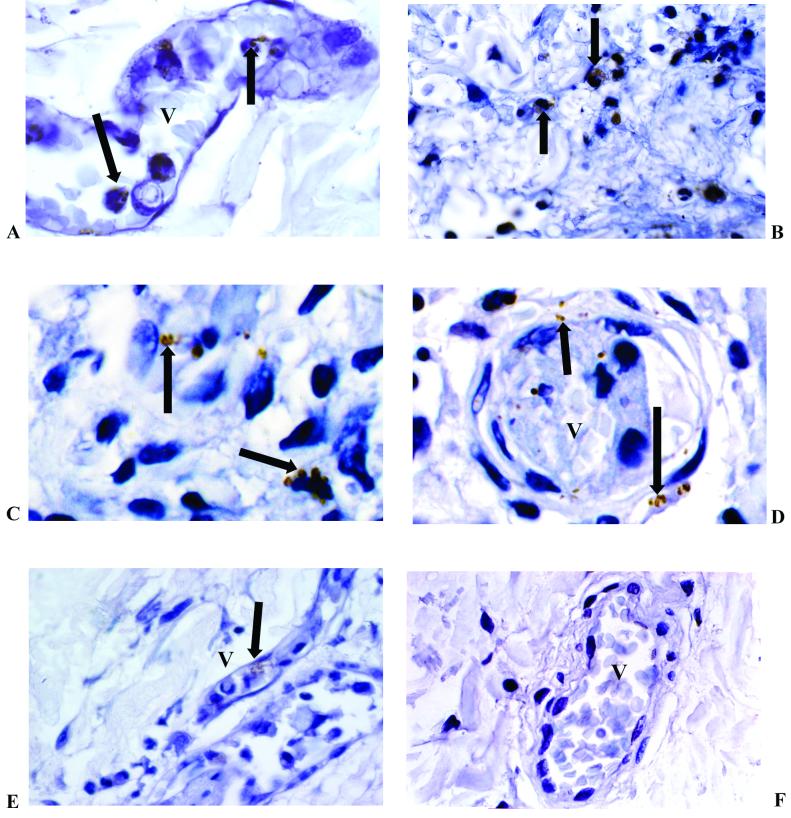
The presence of invading meningococci in the biopsy samples shown in Fig. 1 was confirmed with serogroup-specific anticapsular, serosubtype-specific anti-PorA and antipilin antibodies (Fig. 3). Immunostaining revealed that expression of the capsule and type IV pilin by meningococci was not restricted to any particular location and that meningococci positive for these antigens could be found associated with leukocytes and blood vessels and deep within the dermal interstitium (Fig. 3). Semiquantitative analysis of all five biopsy specimens failed to demonstrate any relationship between microenvironment and expression (Table 1). Further optimizing and antigen retrieval studies to overcome any epitope masking that had resulted from formalin fixation did not elicit additional antigen expression. Double-labeling studies using horseradish peroxidase and diaminobenzidine labeling of meningococcal capsule and alkaline phosphatase and Fast Red labeling of pilin did not show variation in the coexpression of these antigens (results not shown).
FIG. 3.
Immunohistochemical staining of N. meningitidis in skin biopsy samples from patients with meningococcal disease. These examples show capsulated serogroup B visualized in the blood vessel (A) (magnification, ×1,000), inflammatory cells (B) (magnification, ×600), and the interstitium (C) (magnification, ×1,000). PorA stained bacteria in the interstitium and blood vessel (D), and pilin stained meningococci in a small blood vessel (E) (magnification, ×600). A negative control is also shown (F) (magnification, ×400). V, blood vessel. Arrows indicate immunoperoxidase-positive staining of meningococci (brown) with the appropriate specific mouse monoclonal antimeningococcal antibody (nuclei were counterstained in hematoxylin).
TABLE 1.
Semiquantitative analysis of staining of N. meningitidis in skin biopsy sectionsa
| Patient no. (invasive isolate) | Location in biopsy sample | Score by:
|
|||
|---|---|---|---|---|---|
| Expression of:
|
Gram stain | ||||
| Pilin | Capsule | PorA | |||
| 1b (B:NT:P1.15) | Neutrophil | 0 | 0 | 0 | 3+ |
| Blood vessel | 0 | 0 | 0 | 4+ | |
| Interstitium | 0 | 0 | 0 | 2+ | |
| Whole section | 0 | 0 | 0 | 4+ | |
| 2 (B:15:P1.16) | Neutrophil | 4+ | 1+ | 2+ | 1+ |
| Blood vessel | 0 | 1+ | 1+ | 1+ | |
| Interstitium | 1+ | 1+ | 1+ | 1+ | |
| Whole section | 4+ | 2+ | 2+ | 1+ | |
| 3 (B:NT:P1.9) | Neutrophil | 1+ | 2+ | 0 | 4+ |
| Blood vessel | 0 | 3+ | 0 | 0 | |
| Interstitium | 0 | 0 | 0 | 3+ | |
| Whole section | 2+ | 2+ | 0 | 4+ | |
| 4 (B:NT:P1.4) | Neutrophil | 1+ | 1+ | 1+ | 1+ |
| Blood vessel | 1+ | 1+ | 1+ | 1+ | |
| Interstitium | 1+ | 1+ | 1+ | 1+ | |
| Whole section | 1+ | 1+ | 1+ | 2+ | |
| 5 (C:2.2a:P1.2, 5) | Neutrophil | 3+ | 4+ | 3+ | 3+ |
| Blood vessel | 3+ | 1+ | 2+ | 4+ | |
| Interstitium | 1+ | 1+ | 1+ | 0 | |
| Whole section | 4+ | 4+ | 4+ | 4+ | |
For each biopsy sample, the resident meningococci were initially identified by Gram stain and scored and then scored for N. meningitidis pilin, capsule, and PorA expression as detected by immunohistochemistry as follows: 0, no bacteria; 1+, 1 to 20 bacteria; 2+, 21 to 40 bacteria; 3+, 41 to 60 bacteria; 4+, >60 bacteria.
Lack of staining may reflect the intense inflammatory infiltrate seen in this biopsy sample.
Detection of antigen expression, antigen variation, and antigen coexpression by confocal microscopy.
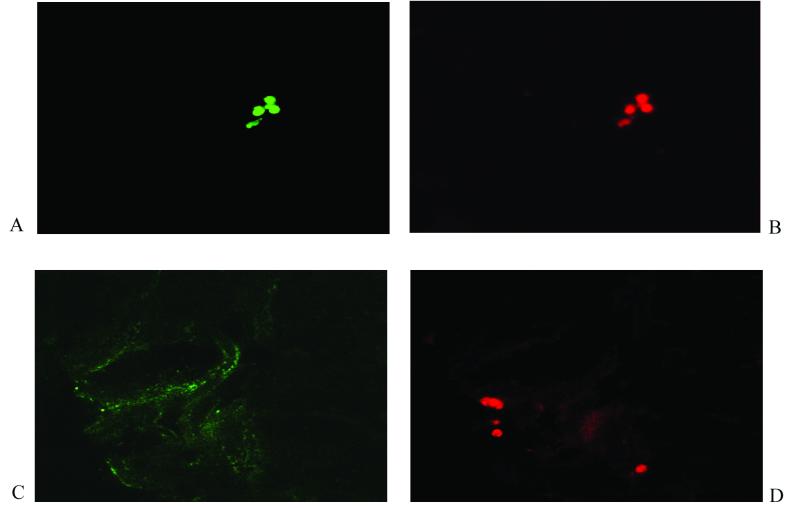
Conventional immunostaining of formalin-fixed material did not exclude the possibility that there is phase variation of meningococcal capsule, PorA, or pilin expression within these biopsy samples. To address this and to explore the possibility that capsule may limit the accessibility of other surface epitopes, we performed double staining of resident meningococci (two specific antibodies) in thick sections of frozen biopsy material. These confocal laser scanning studies revealed microcolonies of meningococci expressing PorA, capsule, and pilin as observed before (Fig. 4). By using Alexa Fluor 488 (green fluorescence)- and Alexa Fluor 594 (red fluorescence)-labeled antibodies, numerous microcolonies containing diplococci of N. meningitidis double stained with anticapsule and antipilin antibodies throughout. However, in addition, in a minority of microcolonies, there was evidence of considerable variation in both capsule and pilin staining (Fig. 4C and D). This occurred both within and between microcolonies in the same section. Compared with these other antigens, PorA staining showed much less variation. The use of thick sections and the autofluorescence of the biopsy material rendered a more detailed comparative analysis of the meningococcal microcolonies within each biopsy unfeasible.
FIG. 4.
Confocal laser scanning microscopy to detect N. meningitidis in skin biopsy samples from patients with meningococcal disease. Seven-micrometer-thick frozen sections were analyzed as sequential 1-μm-thick sections (×1,000). (A and B) A microcolony of N. meningitidis double stained with anticapsule monoclonal antibody detected with Alexa Fluor 488 (green fluorescence) and antipilin monoclonal antibody detected with Alexa Fluor 594 (red fluorescence), respectively. (C and D) A microcolony staining with antipilin monoclonal antibody (red fluorescence) and some background fluorescence but no specific staining with anticapsule monoclonal antibody (green fluorescence), respectively.
DISCUSSION
In this study, we have characterized the pattern of meningococcal surface antigen expression in different cellular microenvironments in vivo. Extending observations made over 20 years ago (23, 47), we have demonstrated that the meningococci that reach the skin invade the deep tissues to form microcolonies that express capsule, PorA, and pilin. These meningococci were frequently in association with an intact endothelium and a cellular infiltrate consisting of neutrophils and monocytes/macrophages.
The vascular endothelium is a key target for the host inflammatory response to meningococcal infection, and the ensuing endothelial damage underlies many of the clinical manifestations associated with this condition (5, 19). The importance of bacterial structure as a determinant of endothelial attachment and invasion and the subsequent cellular dysfunction has been extensively investigated in laboratory models (2, 11, 22, 26, 32, 33, 55-57). Using isogenic mutants which possess a mutation in the polysialyltransferase gene siaD, we have shown that variation in capsule expression modulates bacterial adhesion to host cells, the magnitude of endothelial cell injury, the regulation of tissue factor, and several other important endothelial adhesion molecules (11, 22, 26). The capsule and indeed the majority of the meningococcal surface structures known to modulate interactions with the host have been shown to be phase variable (9, 10, 35, 44, 52, 62). This variation may be rapid (7), rendering analysis of these antigens based on subcultured isolates derived from patients problematic. In order to gain clearer insight into human disease, we have used specific monoclonal antibodies to localize capsule, PorA, and type IV pilin expression in skin biopsy material from children with severe meningococcal sepsis. Immunohistochemistry of the biopsy samples revealed that expression of these antigens was not restricted to any particular microenvironment and that a similar phenotype could be found to be associated with neutrophils, monocytes/macrophages, blood vessels, and the dermal interstitium. These findings suggest that concerted modulation of these antigens may not be critical for the establishment of meningococci in these different environments.
Confocal laser scanning studies confirmed that these microcolonies of meningococci in the skin frequently coexpress capsule and pilin. This supports the possibility that the selection of particular nonencapsulated or nonpiliated phenotypes is not common in these environments. However, there was some variation of these key surface components and positive staining for pilin does not necessarily indicate an intact functioning pilus since some variants may make pilin which does not lead to the assembly of pili (49). Although it is possible that variation in staining observed by confocal microscopy resulted from differences in the accessibility of the antigens to the antibodies used rather than from phase variation, this is unlikely, as the monoclonal antibodies used have appeared robust in a variety of systems, staining intracellular as well as extracellular organisms in our tissue culture model (results not shown). The emergence of variants as a minority population during meningococcal invasive disease may be an important adaptation to an inhospitable host environment (44). Based on the present paradigm of meningococcus-host cell interactions, such variations may enable the organism to further disseminate, accelerating the tissue damage and organ failure commonly associated with the disease.
It has been previously reported that severe meningococcal sepsis is associated with a marked dysfunction of the microvasculature in the skin (14). A striking reduction in the expression of two key molecules implicated in the regulation of thrombosis, thrombomodulin and the endothelial protein C receptor was observed. Ultrastructural studies demonstrated that this reduction is not explained simply by the loss of endothelial cells. Although these endothelial changes may be due to the indirect effects of the inflammatory mediators released in response to meningococcal sepsis, we suggest that the direct effects of adhesive meningococci may also be important (11, 22, 26). The data presented here show capsule expression in multiple environments within the skin, which implies that the complete absence of capsule is not necessary for these interactions to occur. However, small decreases in capsule expression or structure would not have been detected by the available methodology and cannot be ruled out. Two major mechanisms of capsule phase variation are known to take place within meningococci. Site-specific insertion and precise excision of the mobile genetic element IS1301 occurs within the siaA gene of N. meningitidis serogroups expressing a capsule made of polysialic acids (not serogroup A meningococci) (17). The IS1301 insertion has been shown to mediate loss of encapsulation, which resulted in both stronger adherence and increased entry of meningococci into epithelial cells. Frequent phase variation of serogroup B meningococcal capsular polysaccharide also occurs by a reversible +1/−1 frameshift mutation within a poly(dC) repeat, which alters the reading frame of the siaD gene (18). Both of these mechanisms result in the complete loss of capsule expression. In preliminary experiments, DNA has been extracted from frozen skin biopsy samples, followed by amplification, cloning, and sequencing of the poly(dC) repeat region of the siaD gene. Three sequences have been obtained from a single biopsy sample (unpublished results): a poly(dC)6 repeat and a poly(dC)8 repeat which would result in frameshifts that would inactivate the siaD gene, suggesting that there are unencapsulated organisms present within the biopsy sample, and a poly(dC)6 repeat which suggests that, provided siaD is expressed, there are also encapsulated organisms (18).
Despite the recent success of the serogroup C polysaccharide conjugate vaccine (39), an effective serogroup B vaccine remains elusive; however, several serogroup B vaccines based on subcapsular surface antigens are presently under investigation. It has been suggested that stable expression of candidate vaccine antigens is a prerequisite for a vaccine to be effective (53), and there is some evidence that the presence of capsule may reduce the availability of membrane antigens to immunological detection and therefore limit complement-mediated bacteriolysis (31). The double-staining confocal laser scanning microscopy suggests that surface components such as pilin and PorA are available for targeting by host immune defenses either because they project beyond the capsule or because of the open nature of the capsule. We have additional data to suggest that a wider range of surface antigens can be detected by this approach (unpublished results). Comparison of the expression of meningococcal epitopes in situ with those expressed by meningococci derived from blood culture represents a novel approach to the analysis of the wide array of candidate vaccine antigens that has emerged from the sequencing of the meningococcal genome (50).
In summary, we have demonstrated the expression of three key meningococcal virulence factors in the skin lesions of individuals with purpura fulminans in a range of different cellular environments. Although it could be argued that observations made in the skin might not fully reflect events taking place in the circulation and other target tissues, it is likely that similar environments are encountered elsewhere. We speculate that, although not widespread, small but biologically relevant changes in the expression and antigenicity of these molecules may be important in the regulation of host-pathogen interactions, particularly in small subpopulations. The strategy that we have employed will be helpful in investigating invasive bacterial diseases where antigenic and phase variation has a significant impact on virulence and vaccine design.
Acknowledgments
We are grateful to M. Virji and J. S. Kroll for their valuable comments on the manuscript. We gratefully acknowledge the expert help and support of C. A. Ison and the Paediatric Intensive Care clinical team at St. Mary's Hospital.
S.N.F. was funded by the United Kingdom Medical Research Council (MRC). This work was funded by the Meningitis Research Foundation and the Dorset Foundation. The Bristol MRC Cell Imaging Facility is supported by the MRC.
Editor: D. L. Burns
REFERENCES
- 1.Ala'Aldeen, D. A. A., K. R. Neal, K. Ait-Tahar, J. S. Nguyen-Van-Tam, A. English, T. J. Falla, P. M. Hawkey, and R. C. B. Slack. 2000. Dynamics of meningococcal long-term carriage among university students and their implications for mass vaccination. J. Clin. Microbiol. 38:2311-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkness, K. A., B. L. Swisher, E. H. White, E. G. Long, E. P. Ewing, Jr., and F. D. Quinn. 1995. A tissue culture bilayer model to study the passage of Neisseria meningitidis. Infect. Immun. 63:402-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjerknes, R., H. K. Guttormsen, C. O. Solberg, and L. M. Wetzler. 1995. Neisserial porins inhibit human neutrophil actin polymerization, degranulation, opsonin receptor expression, and phagocytosis but prime the neutrophils to increase their oxidative burst. Infect. Immun. 63:160-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjune, G., E. A. Høiby, J. K. Grønnesby, Ø. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A.-K. Lindbak, H. Nøkleby, E. Rosenqvist, L. K. Solberg, O. Closs, J. Eng, L. O. Frøholm, A. Lystad, L. S. Bakketeig, and B. Hareide. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg, P. 1995. Pathogenesis of meningococcal disease, p. 71-114. In K. Cartwright (ed.), Meningococcal disease. John Wiley & Sons Ltd., Chichester, United Kingdom.
- 6.Brown, R. C., and H. C. Hopps. 1973. Staining of bacteria in tissue sections: a reliable gram stain method. Am. J. Clin. Pathol. 60:234-240. [DOI] [PubMed] [Google Scholar]
- 7.Bucci, C., A. Lavitola, P. Salvatore, L. Del Giudice, D. R. Massardo, C. B. Bruni, and P. Alifano. 1999. Hypermutation in pathogenic bacteria: frequent phase variation in meningococci is a phenotypic trait of a specialized mutator biotype. Mol. Cell 3:435-445. [DOI] [PubMed] [Google Scholar]
- 8.de Chabalier, F., M. H. Djingarey, A. Hassane, and J. P. Chippaux. 2000. Meningitis seasonal pattern in Africa and detection of epidemics: a retrospective study in Niger, 1990-98. Trans. R. Soc. Trop. Med. Hyg. 94:664-668. [DOI] [PubMed] [Google Scholar]
- 9.Deghmane, A. E., S. Petit, A. Topilko, Y. Pereira, D. Giorgini, M. Larribe, and M. K. Taha. 2000. Intimate adhesion of Neisseria meningitidis to human epithelial cells is under the control of the crgA gene, a novel LysR-type transcriptional regulator. EMBO J. 19:1068-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vries, F. P., A. van Der Ende, J. P. M. van Putten, and J. Dankert. 1996. Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect. Immun. 64:2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon, G. L. J., R. S. Heyderman, K. Kotovicz, D. L. Jack, U. Voguel, M. Frosch, S. R. Andersen, and N. J. Klein. 1999. Endothelial adhesion molecule expression and its inhibition by recombinant bactericidal/permeability increasing protein (rBPI21) is influenced by the capsulation and LOS structure of Neisseria meningitidis. Infect. Immun. 67:5626-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estabrook, M. M., N. C. Christopher, J. M. Griffiss, C. J. Baker, and R. E. Mandrell. 1992. Sialylation and human neutrophil killing of group C Neisseria meningitidis. J. Infect. Dis. 166:1079-1088. [DOI] [PubMed] [Google Scholar]
- 13.Faust, S. N., R. S. Heyderman, and M. Levin. 2000. Disseminated intravascular coagulation and purpura fulminans secondary to infection. Bailliere's Best Pract. Res. Clin. Haematol. 13:179-197. [DOI] [PubMed] [Google Scholar]
- 14.Faust, S. N., M. Levin, O. Harrison, R. D. Goldin, M. S. Lockhart, S. Kondaveeti, Z. Laszik, C. T. Esmon, and R. S. Heyderman. 2001. Dysfunction of the endothelial protein C activation pathway in severe meningococcal sepsis. N. Engl. J. Med. 345:408-416. [DOI] [PubMed] [Google Scholar]
- 15.Frosch, M., and A. Muller. 1993. Phospholipid substitution of capsular polysaccharides and mechanisms of capsule formation in Neisseria meningitidis. Mol. Microbiol. 8:483-493. [DOI] [PubMed] [Google Scholar]
- 16.Gimbrone, M. A., R. S. Cotran, and J. Folkman. 1974. Human vascular endothelium in culture: growth and DNA synthesis. J. Cell Biol. 60:673-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammerschmidt, S., R. Hilse, J. P. van Putten, R. Gerardy-Schahn, A. Unkmeir, and M. Frosch. 1996. Modulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic element. EMBO J. 15:192-198. [PMC free article] [PubMed] [Google Scholar]
- 18.Hammerschmidt, S., A. Muller, H. Sillmann, M. Muhlenhoff, R. Borrow, A. Fox, J. van Putten, W. D. Zollinger, R. Gerardy-Schahn, and M. Frosch. 1996. Capsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialyltransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal disease. Mol. Microbiol. 20:1211-1220. [DOI] [PubMed] [Google Scholar]
- 19.Heyderman, R. S., and P. Habibi. 2000. Meningococcal infections of the skin, p. 384-394. In J. Harper, A. Oranje, and N. Prose (ed.), Textbook of paediatric dermatology. Blackwell Science, Oxford, England.
- 20.Heyderman, R. S., C. A. Ison, M. Peakman, M. Levin, and N. J. Klein. 1999. Neutrophil response to Neisseria meningitidis: inhibition of adhesion molecule expression and phagocytosis by recombinant bactericidal/permeability-increasing protein (rBPI21). J. Infect. Dis. 179:1288-1292. [DOI] [PubMed] [Google Scholar]
- 21.Heyderman, R. S., and N. J. Klein. 2001. Interactions of meningococci with endothelium, p. 649-661. In A. J. Pollard and M. C. Maiden (ed.), Methods in molecular medicine: Meningococcal disease. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 22.Heyderman, R. S., N. J. Klein, C. A. Ison, M. Peakman, O. A. Daramola, S. Hammerschmidt, M. Frosch, and M. Levin. 1997. The induction of human endothelial tissue factor expression by Neisseria meningitidis. Microb. Pathog. 22:265-274. [DOI] [PubMed] [Google Scholar]
- 23.Hill, W., and T. D. Kinney. 1947. The cutaneous lesions in acute meningococcemia. JAMA 134:513-518. [DOI] [PubMed] [Google Scholar]
- 24.Jones, G. R., M. Christodoulides, J. L. Brooks, A. R. Miller, K. A. Cartwright, and J. E. Heckels. 1998. Dynamics of carriage of Neisseria meningitidis in a group of military recruits: subtype stability and specificity of the immune response following colonization. J. Infect. Dis. 178:451-459. [DOI] [PubMed] [Google Scholar]
- 25.Kallstrom, H., M. K. Liszewski, J. P. Atkinson, and A. B. Jonsson. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic neisseria. Mol. Microbiol. 25:639-647. [DOI] [PubMed] [Google Scholar]
- 26.Klein, N. J., C. A. Ison, M. Peakman, M. Levin, S. Hammerschmidt, M. Frosch, and R. S. Heyderman. 1996. The influence of capsulation and lipooligosaccharide structure on neutrophil adhesion molecule expression and endothelial injury by Neisseria meningitidis. J. Infect. Dis. 173:172-179. [DOI] [PubMed] [Google Scholar]
- 27.McNeil, G., and M. Virji. 1997. Phenotypic variants of meningococci and their potential in phagocytic interactions: the influence of opacity proteins, pili, PilC and surface sialic acids. Microb. Pathog. 22:295-304. [DOI] [PubMed] [Google Scholar]
- 28.McNeil, G., M. Virji, and E. R. Moxon. 1994. Interactions of Neisseria meningitidis with human monocytes. Microb. Pathog. 16:153-163. [DOI] [PubMed] [Google Scholar]
- 29.Merz, A. J., C. A. Enns, and M. So. 1999. Type IV pili of pathogenic neisseriae elicit cortical plaque formation in epithelial cells. Mol. Microbiol. 32:1316-1332. [DOI] [PubMed] [Google Scholar]
- 30.Merz, A. J., D. B. Rifenbery, C. G. Arvidson, and M. So. 1996. Traversal of a polarized epithelium by pathogenic Neisseriae: facilitation by type IV pili and maintenance of epithelial barrier function. Mol. Med. 2:745-754. [PMC free article] [PubMed] [Google Scholar]
- 31.Moe, G. R., S. Tan, and D. M. Granoff. 1999. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect. Immun. 67:5664-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muenzner, P., C. Dehio, T. Fujiwara, M. Achtman, T. F. Meyer, and S. D. Gray-Owen. 2000. Carcinoembryonic antigen family receptor specificity of Neisseria meningitidis Opa variants influences adherence to and invasion of proinflammatory cytokine-activated endothelial cells. Infect. Immun. 68:3601-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nassif, X., J. L. Beretti, J. Lowy, P. Stenberg, P. O'Gaora, J. Pfeifer, S. Normark, and M. So. 1994. Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proc. Natl. Acad. Sci. USA 91:3769-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nassif, X., C. Pujol, P. Morand, and E. Eugene. 1999. Interactions of pathogenic neisseria with host cells. Is it possible to assemble the puzzle? Mol. Microbiol. 32:1124-1132. [DOI] [PubMed] [Google Scholar]
- 35.Patrick, C. C., G. T. Furuta, M. Edwards, M. Estabrook, M. S. Blake, and C. J. Baker. 1993. Variation in phenotypic expression of the Opa outer membrane protein and lipooligosaccharide of Neisseria meningitidis serogroup C causing periorbital cellulitis and bacteremia. Clin. Infect. Dis. 16:523-527. [DOI] [PubMed] [Google Scholar]
- 36.Perkins, B. A., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A. Hoiby, E. Rosenqvist, J. Holst, H. Nokleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapczynski, E. Tikhomirov, J. D. Wenger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683-691. [DOI] [PubMed] [Google Scholar]
- 37.Pujol, C., E. Eugene, M. Marceau, and X. Nassif. 1999. The meningococcal PilT protein is required for induction of intimate attachment to epithelial cells following pilus-mediated adhesion. Proc. Natl. Acad. Sci. USA 96:4017-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramsay, M., E. Kaczmarski, M. Rush, R. Mallard, P. Farrington, and J. White. 1997. Changing patterns of case ascertainment and trends in meningococcal disease in England and Wales. Commun. Dis. Rep. Rev. 7:R49-R54. [PubMed] [Google Scholar]
- 39.Ramsay, M. E., N. Andrews, E. B. Kaczmarski, and E. Miller. 2001. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 357:195-196. [DOI] [PubMed] [Google Scholar]
- 40.Read, R. C., A. Fox, K. Miller, T. Gray, N. Jones, R. Borrows, D. M. Jones, and R. G. Finch. 1995. Experimental infection of human nasal mucosal explants with Neisseria meningitidis. J. Med. Microbiol. 42:353-361. [DOI] [PubMed] [Google Scholar]
- 41.Richter-Dahlfors, A., A. M. Buchan, and B. B. Finlay. 1997. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 186:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, L. Lefkowitz, M. L. Cartter, R. Danila, P. Cieslak, K. A. Shutt, T. Popovic, A. Schuchat, L. H. Harrison, and A. L. Reingold. 1999. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J. Infect. Dis. 180:1894-1901. [DOI] [PubMed] [Google Scholar]
- 43.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 44.Saunders, N. J., A. C. Jeffries, J. F. Peden, D. W. Hood, H. Tettelin, R. Rappuoli, and E. R. Moxon. 2000. Repeat-associated phase variable genes in the complete genome sequence of Neisseria meningitidis strain MC58. Mol. Microbiol. 37:207-215. [DOI] [PubMed] [Google Scholar]
- 45.Schwan, E. T., B. D. Robertson, H. Brade, and J. P. van Putten. 1995. Gonococcal rfaF mutants express Rd2 chemotype LPS and do not enter epithelial host cells. Mol. Microbiol. 15:267-275. [DOI] [PubMed] [Google Scholar]
- 46.Sierra, G. V., H. C. Campa, N. M. Varcacel, I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH (Natl. Inst. Public Health) Ann. (Oslo) 14:195-207. [PubMed] [Google Scholar]
- 47.Soto, M. N., B. Langer, S. Hoshino-Shimizu, and T. de Brito. 1976. Pathogenesis of cutaneous lesions in acute meningococcemia in humans: light, immunofluorescent, and electron microscopic studies of skin biopsy specimens. J. Infect. Dis. 133:506-514. [DOI] [PubMed] [Google Scholar]
- 48.Suker, J., I. M. Feavers, and M. C. Maiden. 1996. Monoclonal antibody recognition of members of the meningococcal P1.10 variable region family: implications for serological typing and vaccine design. Microbiology 142:63-69. [DOI] [PubMed] [Google Scholar]
- 49.Taha, M. K., P. C. Morand, Y. Pereira, E. Eugene, D. Giorgini, M. Larribe, and X. Nassif. 1998. Pilus-mediated adhesion of Neisseria meningitidis: the essential role of cell contact-dependent transcriptional upregulation of the PilC1 protein. Mol. Microbiol. 28:1153-1163. [DOI] [PubMed] [Google Scholar]
- 50.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 51.Thomson, A. P. J., J. A. Sills, and A. Hart. 1991. Validation of the Glasgow meningococcal septicaemia prognostic score: a 10 year retrospective survey. Crit. Care Med. 19:26-30. [DOI] [PubMed] [Google Scholar]
- 52.Tinsley, C. R., and J. E. Heckels. 1986. Variation in the expression of pili and outer membrane protein by Neisseria meningitidis during the course of meningococcal infection. J. Gen. Microbiol. 132:2483-2490. [DOI] [PubMed] [Google Scholar]
- 53.van der Ende, A., C. T. Hopman, and J. Dankert. 2000. Multiple mechanisms of phase variation of PorA in Neisseria meningitidis. Infect. Immun. 68:6685-6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Putten, J., J. F. Weel, and H. U. Grassme. 1994. Measurements of invasion by antibody labeling and electron microscopy. Methods Enzymol. 236:420-437. [DOI] [PubMed] [Google Scholar]
- 55.Virji, M., C. Alexandrescu, D. J. Ferguson, J. R. Saunders, and E. R. Moxon. 1992. Variations in the expression of pili: the effect on adherence of Neisseria meningitidis to human epithelial and endothelial cells. Mol. Microbiol. 6:1271-1279. [DOI] [PubMed] [Google Scholar]
- 56.Virji, M., K. Makepeace, D. J. Ferguson, M. Achtman, and E. R. Moxon. 1993. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol. Microbiol. 10:499-510. [DOI] [PubMed] [Google Scholar]
- 57.Virji, M., K. Makepeace, D. J. Ferguson, M. Achtman, J. Sarkari, and E. R. Moxon. 1992. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol. Microbiol. 6:2785-2795. [DOI] [PubMed] [Google Scholar]
- 58.Virji, M., K. Makepeace, and E. R. Moxon. 1994. Distinct mechanisms of interactions of Opc-expressing meningococci at apical and basolateral surfaces of human endothelial cells; the role of integrins in apical interactions. Mol. Microbiol. 14:173-184. [DOI] [PubMed] [Google Scholar]
- 59.Virji, M., K. Makepeace, I. R. Peak, D. J. Ferguson, M. P. Jennings, and E. R. Moxon. 1995. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol. Microbiol. 18:741-754. [DOI] [PubMed] [Google Scholar]
- 60.Virji, M., K. Makepeace, I. R. Peak, D. J. Ferguson, and E. R. Moxon. 1996. Pathogenic mechanisms of Neisseria meningitidis. Ann. N. Y. Acad. Sci. 797:273-276. [DOI] [PubMed] [Google Scholar]
- 61.Virji, M., S. M. Watt, S. Barker, K. Makepeace, and R. Doyonnas. 1996. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol. Microbiol. 22:929-939. [DOI] [PubMed] [Google Scholar]
- 62.Vogel, U., H. Claus, and M. Frosch. 2000. Rapid serogroup switching in Neisseria meningitidis. N. Engl. J. Med. 342:219-220. [DOI] [PubMed] [Google Scholar]
- 63.Wall, R. A., H. A. Davies, and S. P. Borriello. 1989. Epitopes of serogroup B Neisseria meningitidis analysed in vitro and directly from cerebrospinal fluid. FEMS Microbiol. Lett. 53:129-135. [DOI] [PubMed] [Google Scholar]