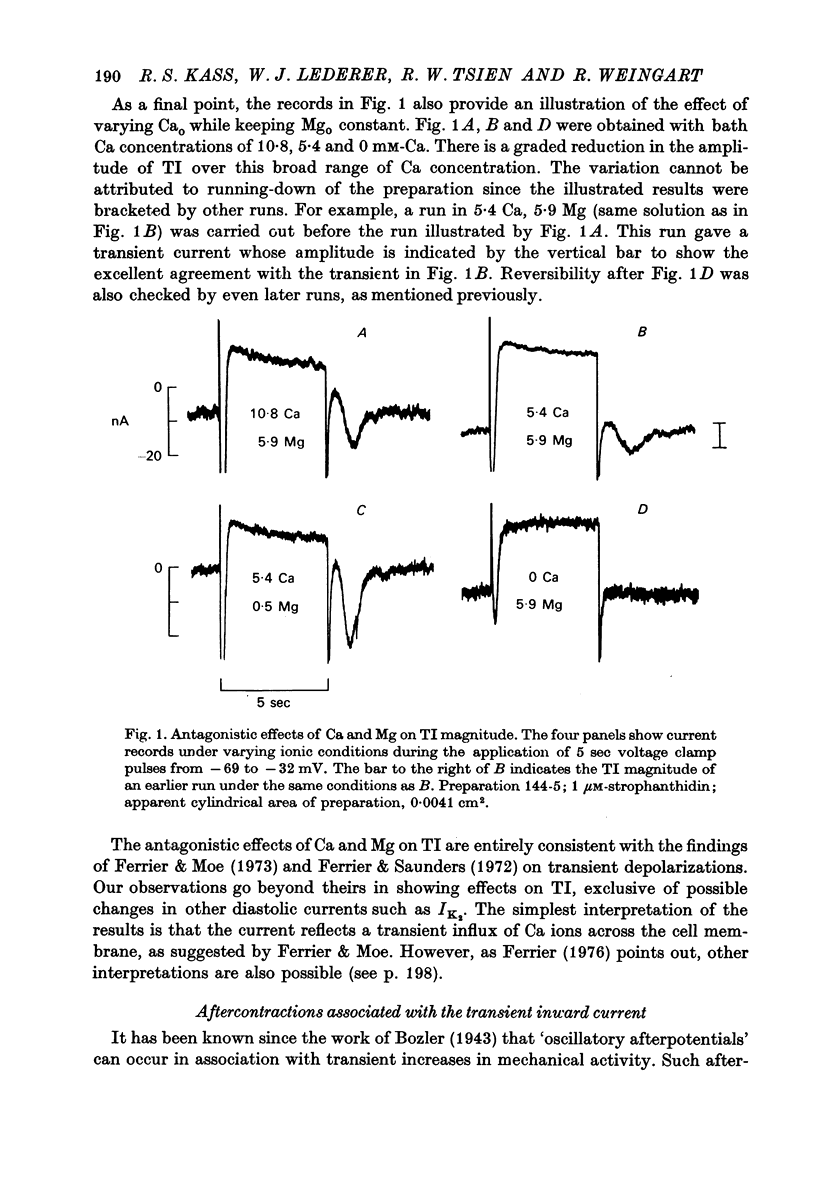
Abstract
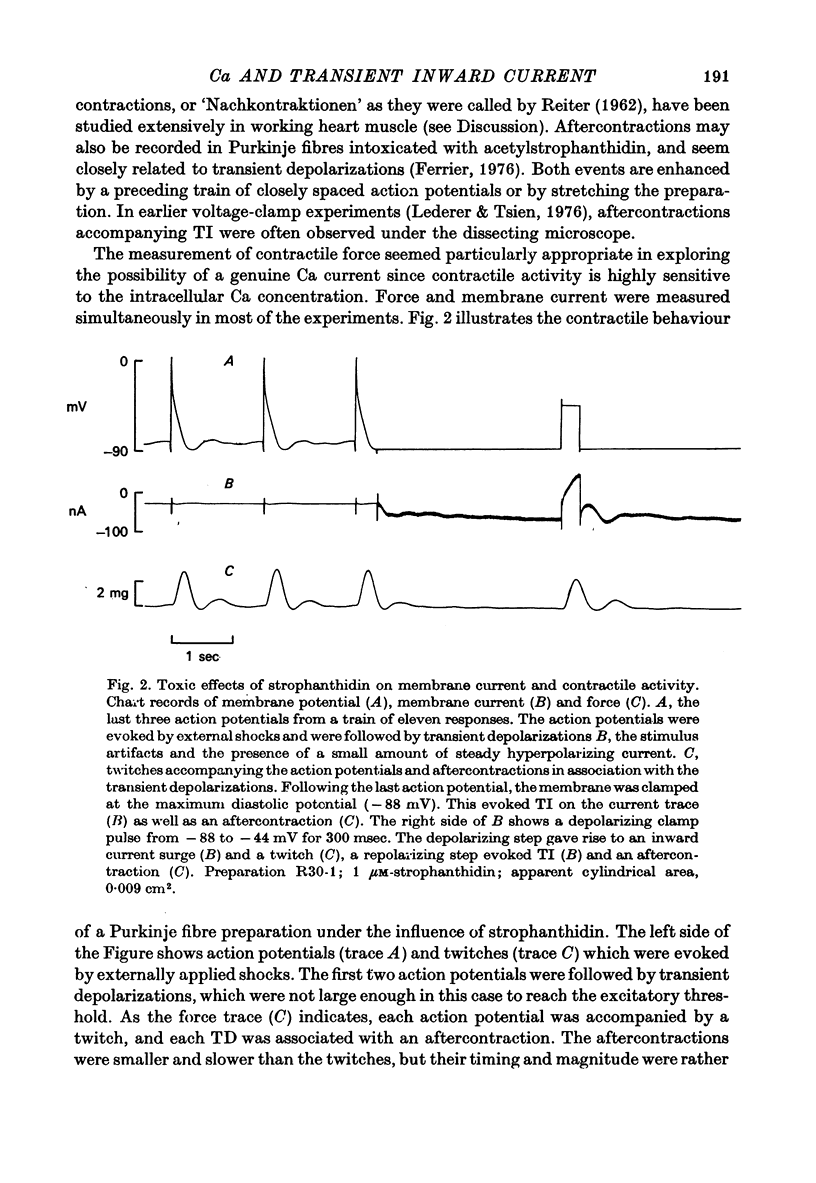
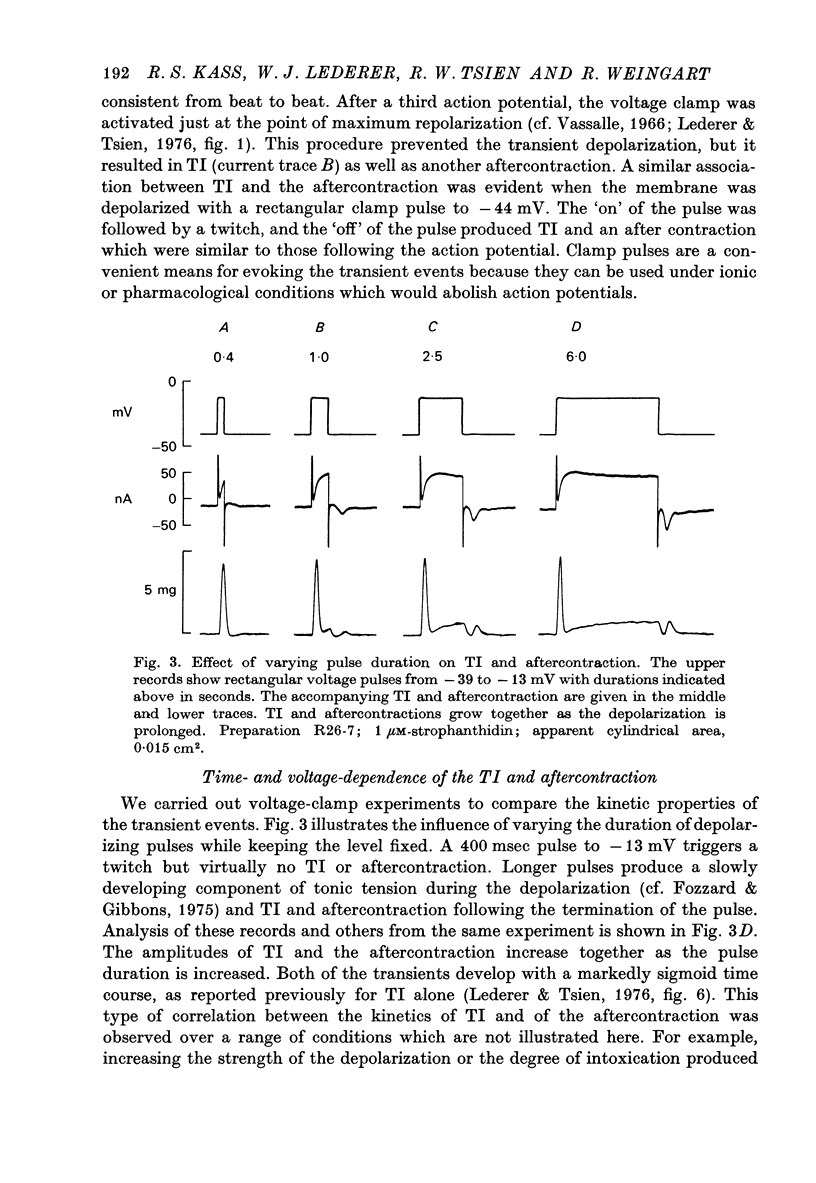
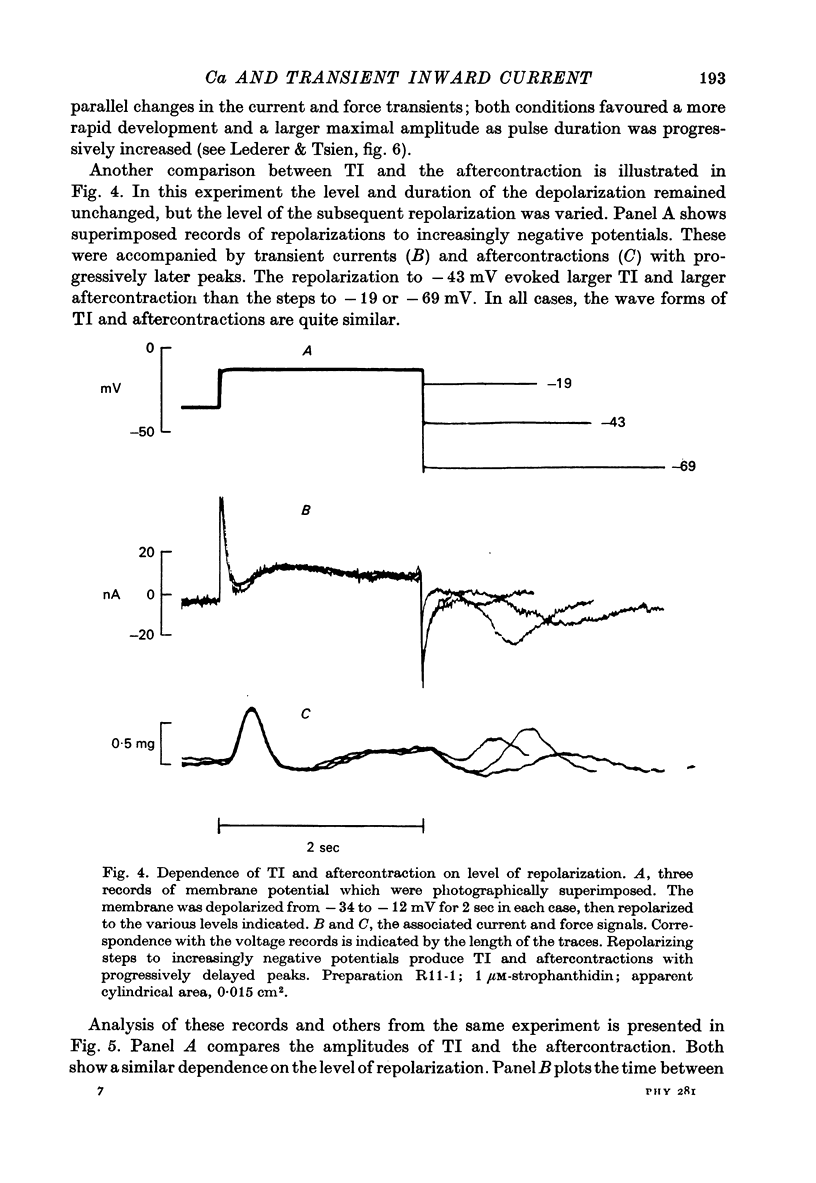
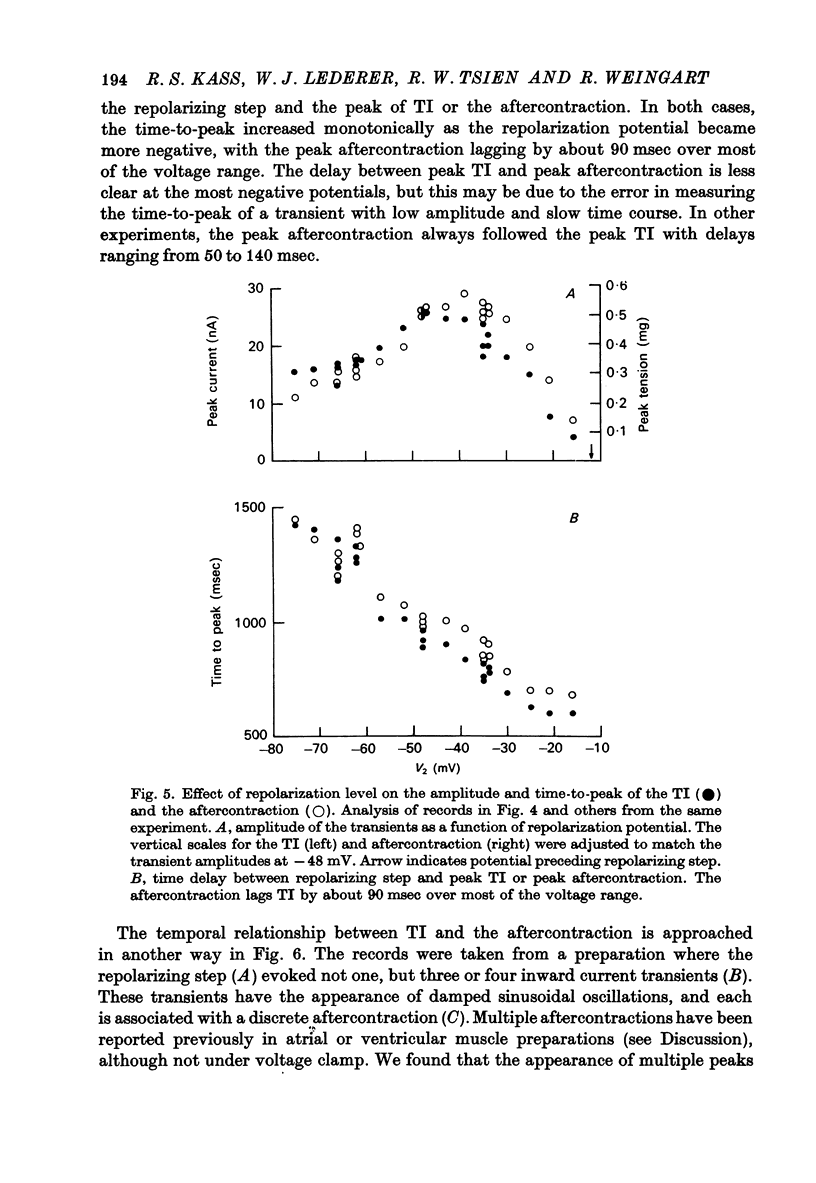
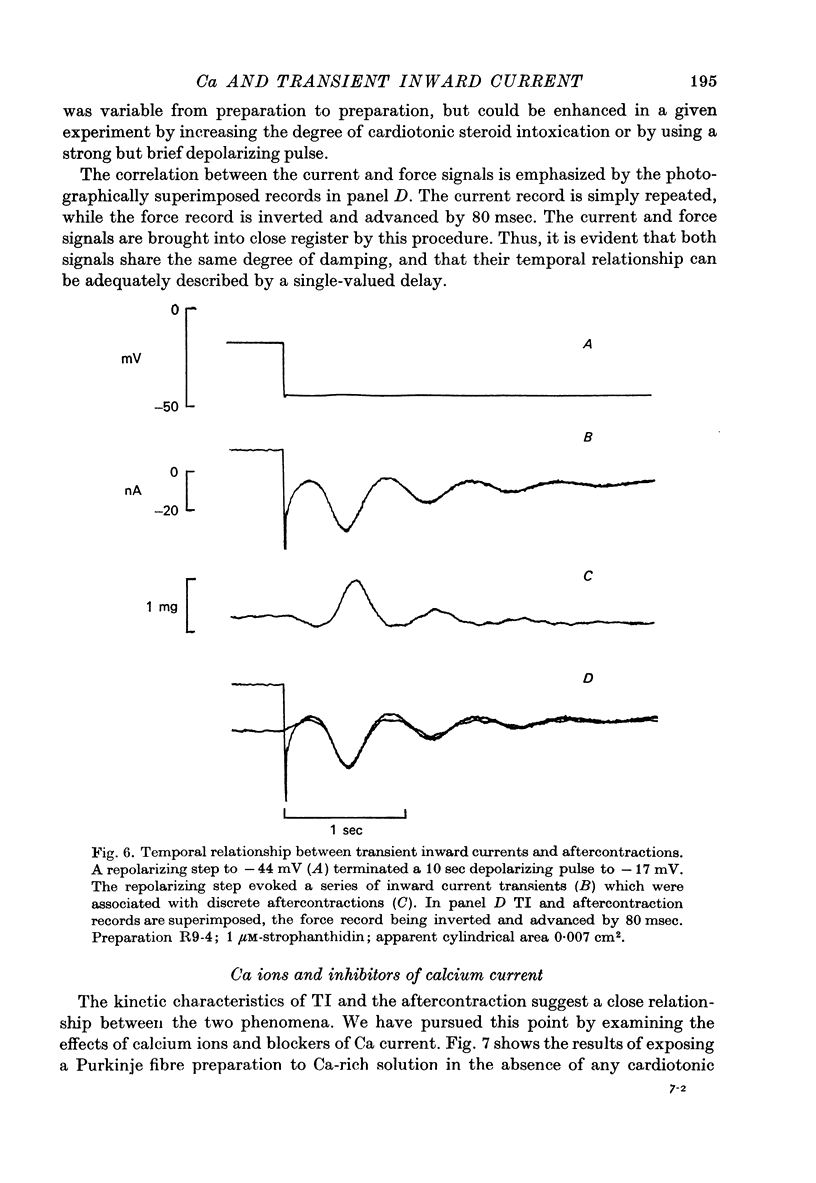
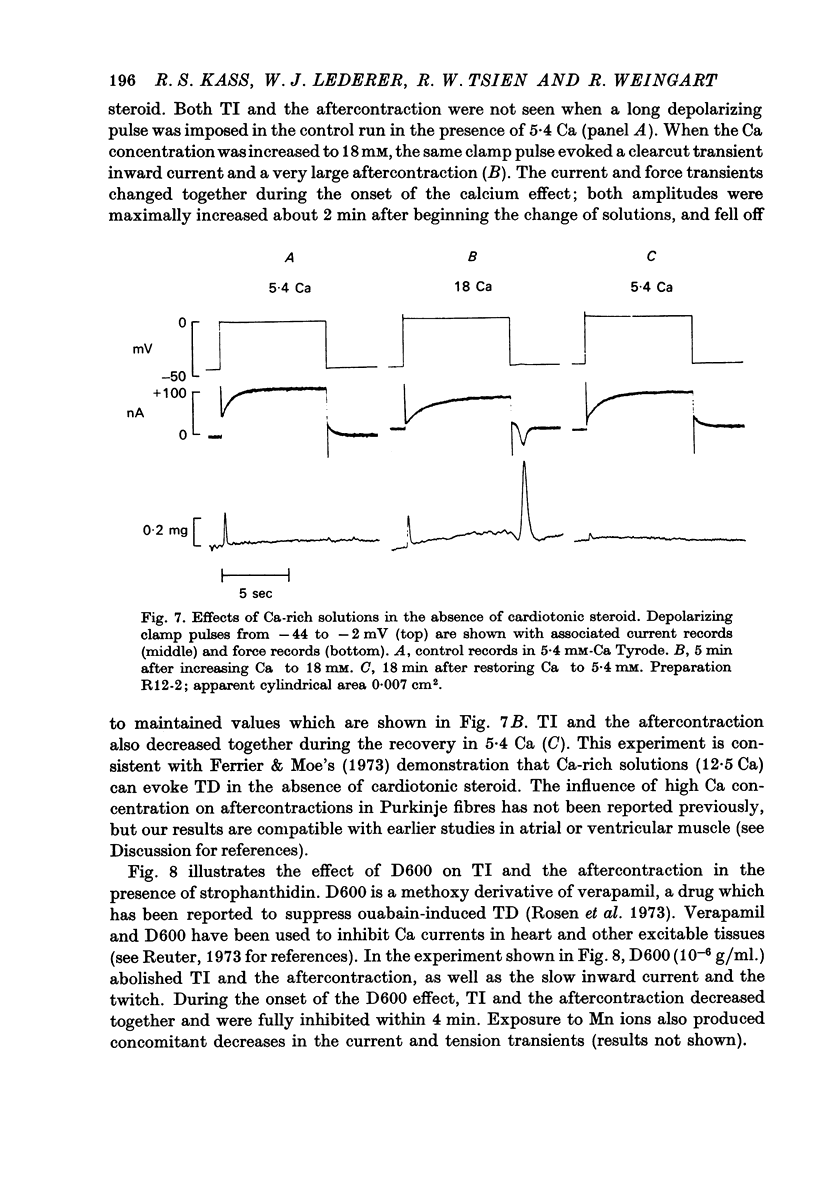
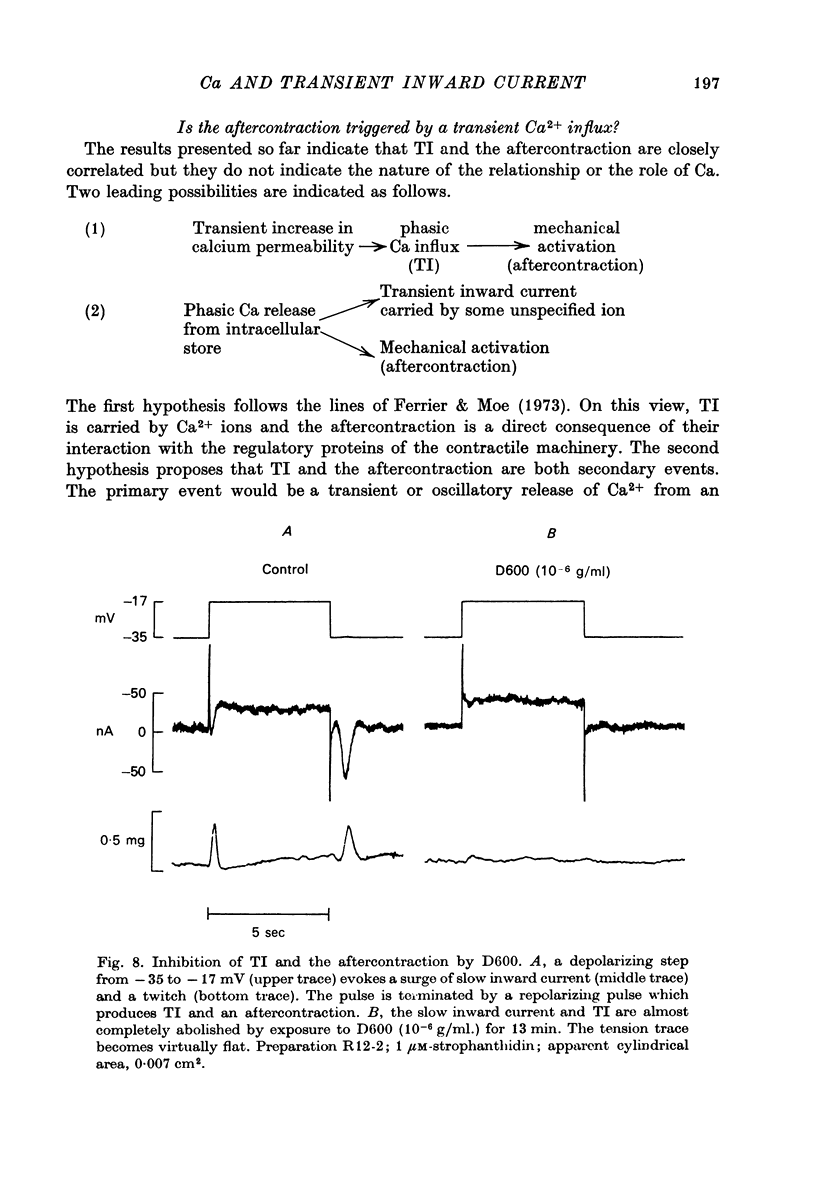
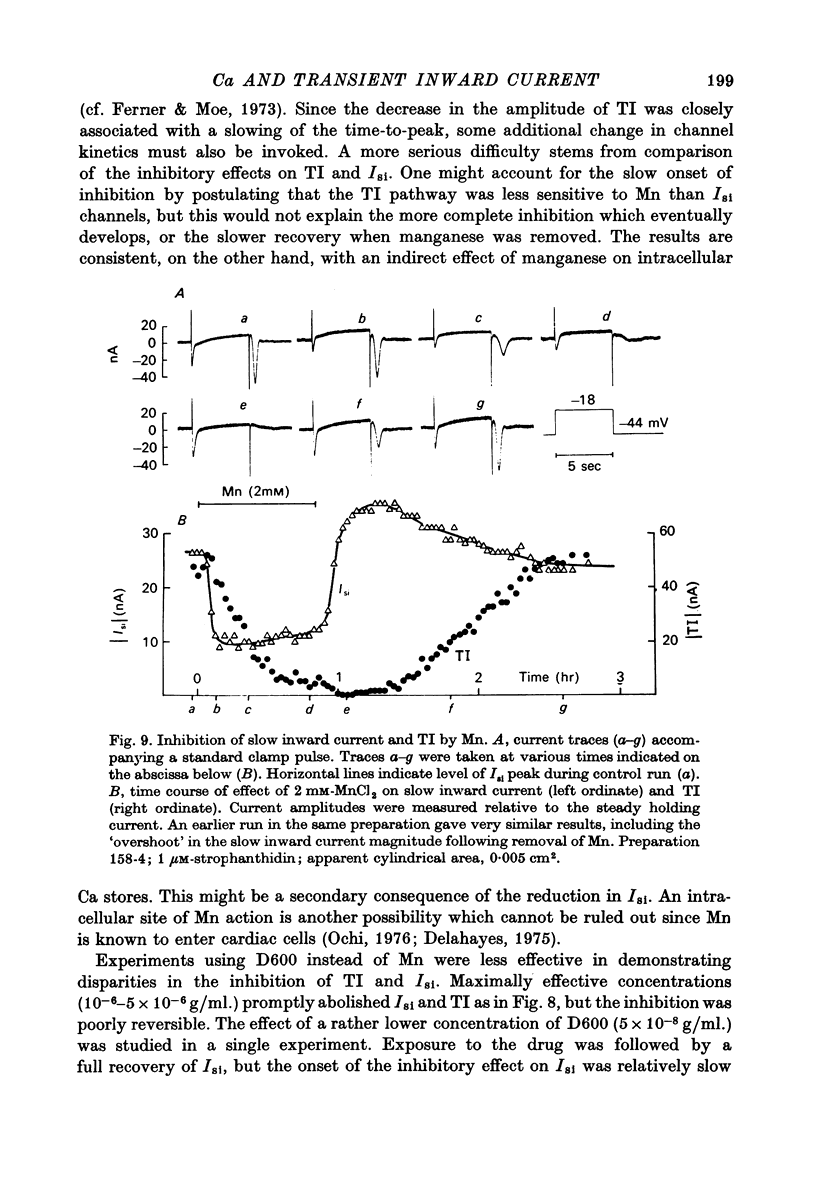
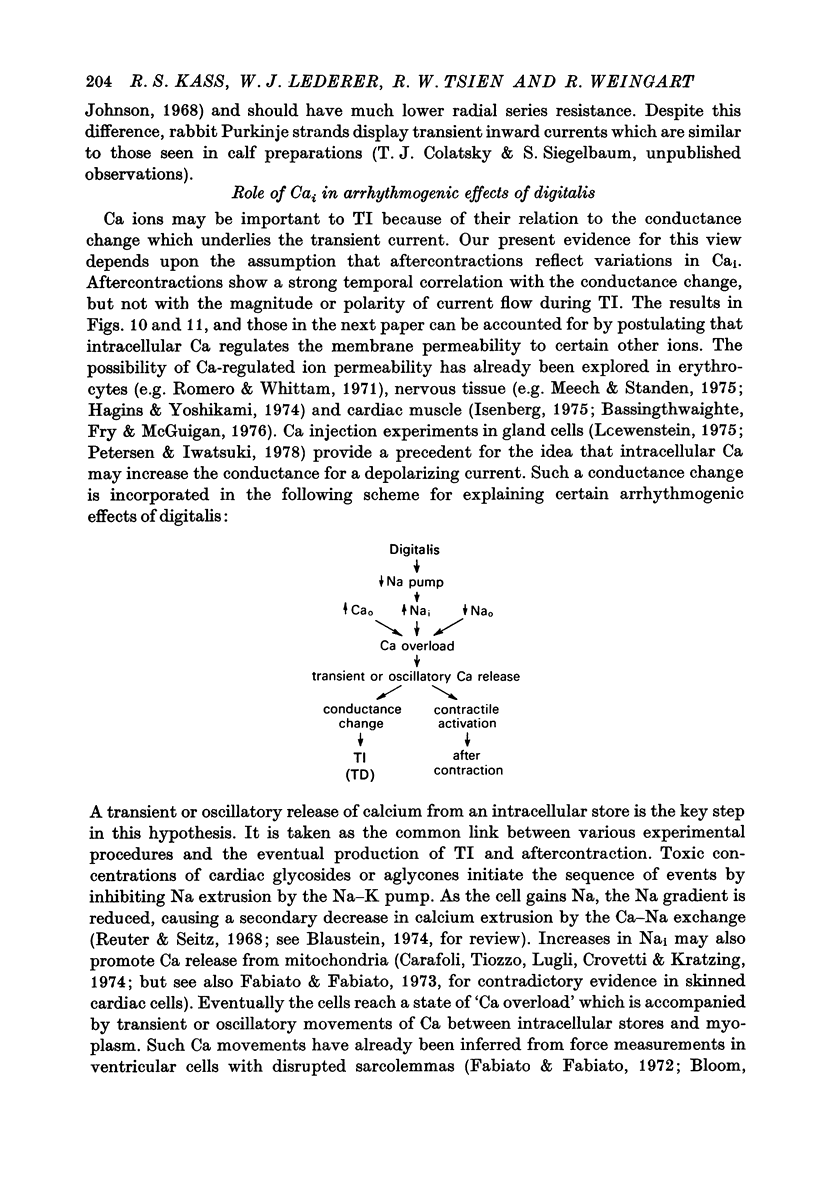
1. Under the influence of strophantidin, Purkinje fibres exhibit transient inward current (TI) which contributes to arrhythmogenic activity. Voltage-clamp experiments were carried out to study the role of Ca ions in this phenomenon. 2. The amplitude of TI varied directly with the extracellular Ca concentration, CaO. Magnesium ions had an antagonistic effect. 3. TI was closely associated with a phasic increase in force ("aftercontraction"). Like TI, the aftercontraction was evoked by a preceding action potential or by the break of a strong depolarizing pulse. 4. TI and the aftercontraction displayed similar wave forms although peak current preceded peak force by 50--100 msec. Both transients were enhanced by increasing the strength or duration of the preceding depolarization pulse. Both events were slowed as the potential level following the pulse was displaced in the negative direction. 5. TI and the aftercontraction could be evoked in the absence of cardiotonic steroids by strongly elevating CaO. 6. Additional experiments were carried out to test the hypothesis the TI reflects an influx of Ca2+ ions. Moninhibited TI but the developed and removal of the inbibition lagged far behind the effects on the slow inward current. 7. TI could be suppressed and eventually inverted by varying the membrane potential in the positive direction. The inversion potential averaged -5mV and was not consistent with a Ca-specific pathway. The aftercontraction was more closely related to the phasic conductance change underlying the current than to thecurrent flow itself. 8. The results are consistent with the idea that an oscillatory release of Ca from an intracellular store is the primary event underlying both the aftercontraction and the conductance change which generates TI. Digitalis intoxication or very high CaO may promote such events by elevating intracellular Ca levels.
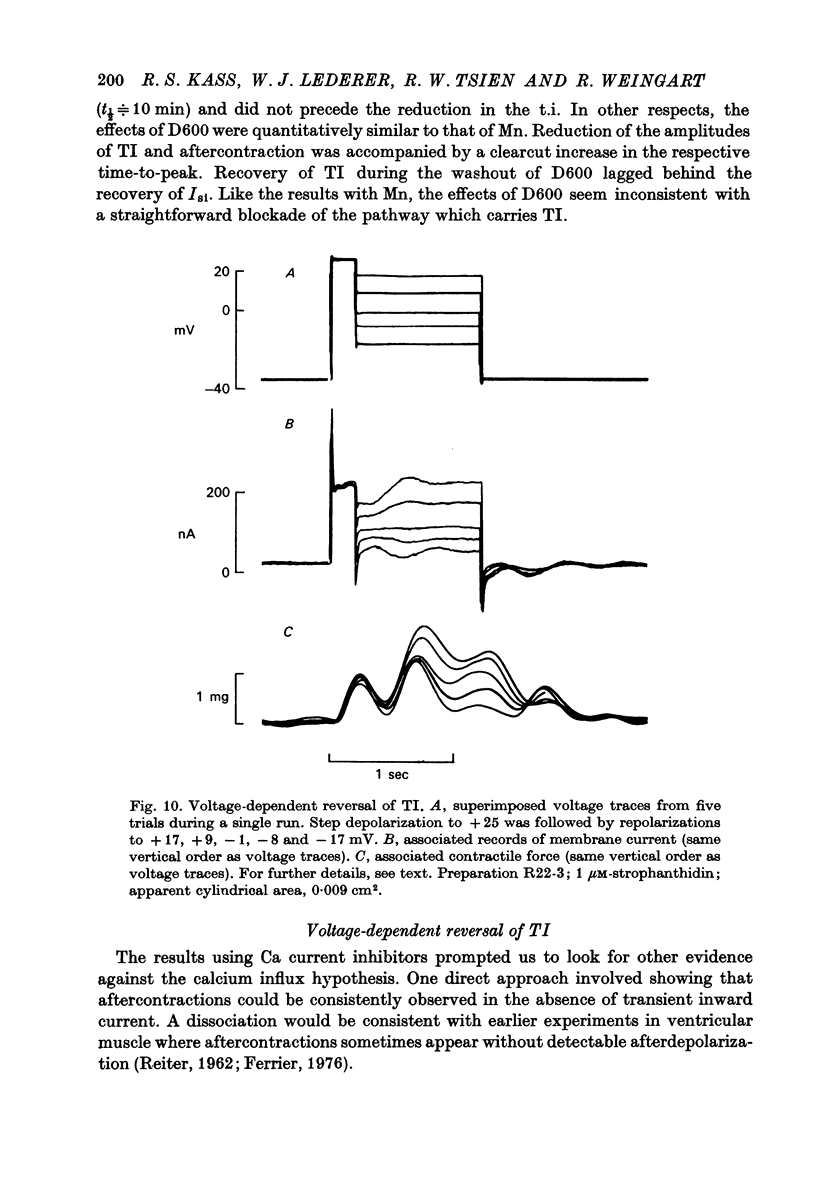
Full text
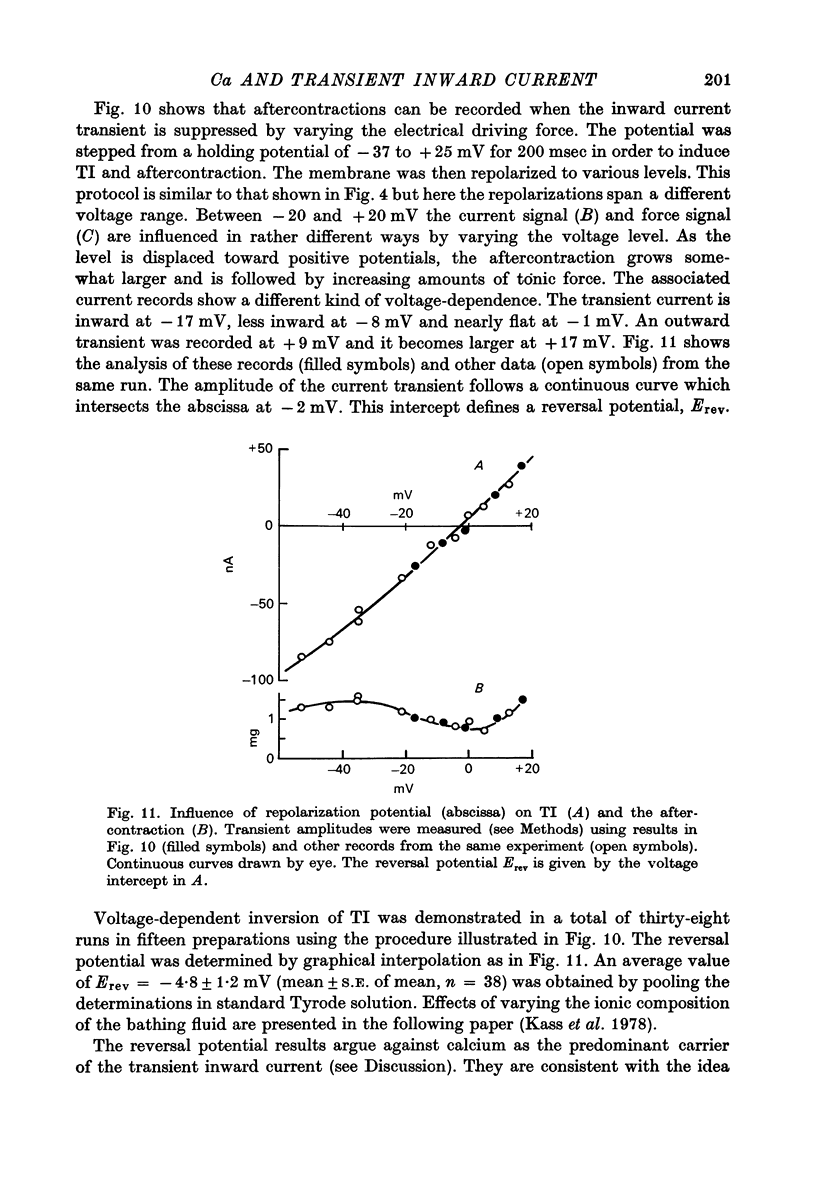
PDF



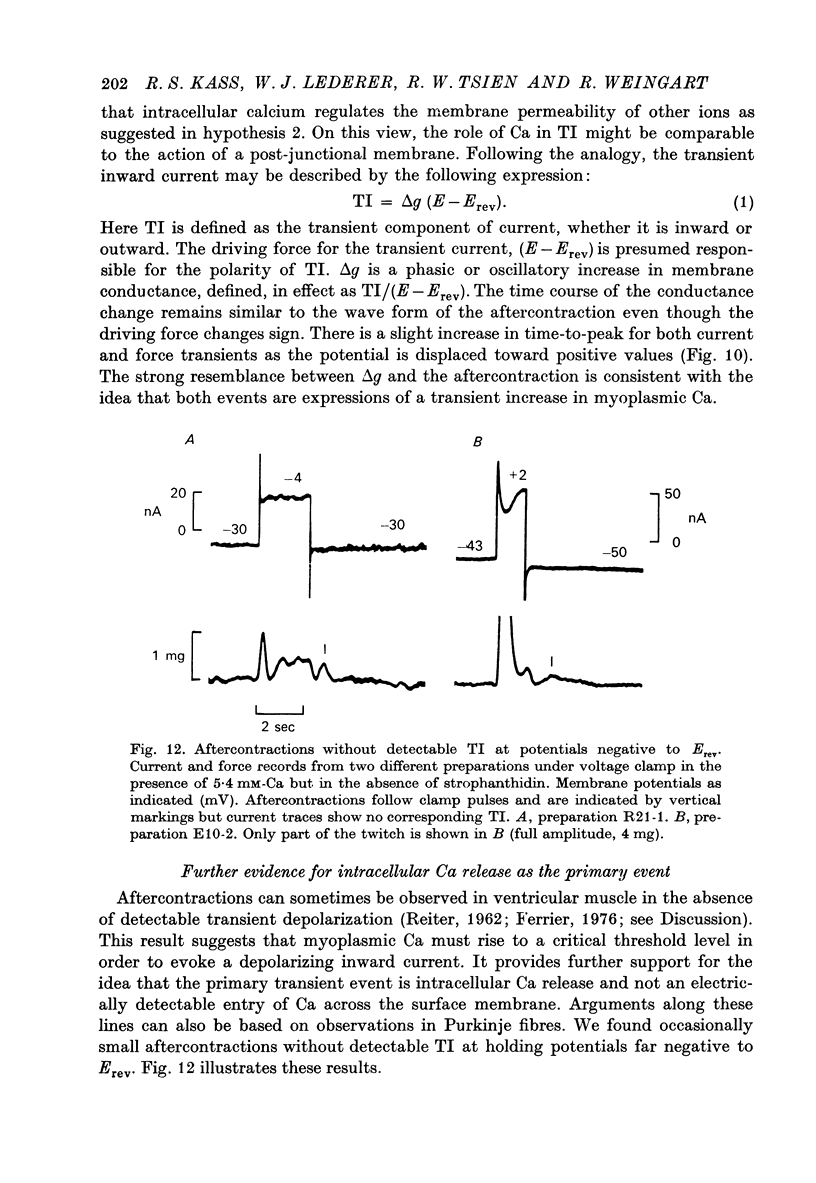





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassingthwaighte J. B., Fry C. H., McGuigan J. A. Relationship between internal calcium and outward current in mammalian ventricular muscle; a mechanism for the control of the action potential duration? J Physiol. 1976 Oct;262(1):15–37. doi: 10.1113/jphysiol.1976.sp011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Bloom S., Brady A. J., Langer G. A. Calcium metabolism and active tension in mechanically disaggregated heart muscle. J Mol Cell Cardiol. 1974 Apr;6(2):137–147. doi: 10.1016/0022-2828(74)90017-0. [DOI] [PubMed] [Google Scholar]
- Bravený P., Sumbera J., Kruta V. After-contractions and restitution of contractility in the isolated guinea-pig auricles. Arch Int Physiol Biochim. 1966 Feb;74(1):169–178. doi: 10.3109/13813456609059899. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Tiozzo R., Lugli G., Crovetti F., Kratzing C. The release of calcium from heart mitochondria by sodium. J Mol Cell Cardiol. 1974 Aug;6(4):361–371. doi: 10.1016/0022-2828(74)90077-7. [DOI] [PubMed] [Google Scholar]
- DECK K. A., KERN R., TRAUTWEIN W. VOLTAGE CLAMP TECHNIQUE IN MAMMALIAN CARDIAC FIBRES. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jun 9;280:50–62. doi: 10.1007/BF00412615. [DOI] [PubMed] [Google Scholar]
- Davis L. D. Effect of changes in cycle length on diastolic depolarization produced by ouabain in canine Purkinje fibers. Circ Res. 1973 Feb;32(2):206–214. doi: 10.1161/01.res.32.2.206. [DOI] [PubMed] [Google Scholar]
- Delahayes J. F. Depolarization-induced movement of Mn 2 cations across the cell membrane in the guinea pig myocardium. Circ Res. 1975 Jun;36(6):713–718. doi: 10.1161/01.res.36.6.713. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Activation of skinned cardiac cells. Subcellular effects of cardioactive drugs. Eur J Cardiol. 1973 Dec;1(2):143–155. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res. 1972 Sep;31(3):293–307. doi: 10.1161/01.res.31.3.293. [DOI] [PubMed] [Google Scholar]
- Ferrier G. R. Digitalis arrhythmias: role of oscillatory afterpotentials. Prog Cardiovasc Dis. 1977 May-Jun;19(6):459–474. doi: 10.1016/0033-0620(77)90010-x. [DOI] [PubMed] [Google Scholar]
- Ferrier G. R., Moe G. K. Effect of calcium on acetylstrophanthidin-induced transient depolarizations in canine Purkinje tissue. Circ Res. 1973 Nov;33(5):508–515. doi: 10.1161/01.res.33.5.508. [DOI] [PubMed] [Google Scholar]
- Ferrier G. R., Saunders J. H., Mendez C. A cellular mechanism for the generation of ventricular arrhythmias by acetylstrophanthidin. Circ Res. 1973 May;32(5):600–609. doi: 10.1161/01.res.32.5.600. [DOI] [PubMed] [Google Scholar]
- Ferrier G. R. The effects of tension on acetylstrophanthidin-induced transient depolarizations and aftercontractions in canine myocardial and Purkinje tissues. Circ Res. 1976 Mar;38(3):156–162. doi: 10.1161/01.res.38.3.156. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., Gibbons W. R. Action potential and contraction of heart muscle. Am J Cardiol. 1973 Feb;31(2):182–192. doi: 10.1016/0002-9149(73)91031-x. [DOI] [PubMed] [Google Scholar]
- Ghani M. F., Smith J. R. The effectiveness of magnesium chloride in the treatment of ventricular tachyarrhythmias due to digitalis intoxication. Am Heart J. 1974 Nov;88(5):621–626. doi: 10.1016/0002-8703(74)90248-8. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Pott L. Spontaneous tension oscillations in guinea-pig atrial trabeculae. Pflugers Arch. 1975 Jul 9;358(1):11–25. doi: 10.1007/BF00584566. [DOI] [PubMed] [Google Scholar]
- Goshima K. Arrhythmic movements of myocardial cells in culture and their improvement with antiarrhythmic drugs. J Mol Cell Cardiol. 1976 Mar;8(3):217–238. doi: 10.1016/0022-2828(76)90038-9. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Proceedings: A role for Ca2+ in excitation of retinal rods and cones. Exp Eye Res. 1974 Mar;18(3):299–305. doi: 10.1016/0014-4835(74)90157-2. [DOI] [PubMed] [Google Scholar]
- Hogan P. M., Wittenberg S. M., Klocke F. J. Relationship of stimulation frequency to automaticity in the canine Purkinje fiber during ouabain administration. Circ Res. 1973 Mar;32(3):377–384. doi: 10.1161/01.res.32.3.377. [DOI] [PubMed] [Google Scholar]
- Isnberg G. Is potassium conductance of cardiac Purkinje fibres controlled by (Ca2+)? Nature. 1975 Jan 24;253(5489):273–274. doi: 10.1038/253273a0. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Katzung B. G. Simultaneously recorded oscillations in membrane potential and isometric contractile force from cardiac muscle. Nature. 1968 Mar 9;217(5132):961–963. doi: 10.1038/217961a0. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Sommer J. R. A strand of cardiac muscle. Its ultrastructure and the electrophysiological implications of its geometry. J Cell Biol. 1967 Apr;33(1):103–129. doi: 10.1083/jcb.33.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMANN R., FLECKENSTEIN A., ANTONI H. URSACHEN UND AUSLOESUNGSBEDINGUNGEN VON MYOKARD-KONTRAKTIONEN OHNE REGULAERES AKTIONSPOTENTIAL. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963 Nov 29;278:435–446. [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W., Weingart R. Ionic basis of transient inward current induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:209–226. doi: 10.1113/jphysiol.1978.sp012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Tsien R. W. Proceedings: Transient inward current underlying strophanthidin's enhancement of pace-maker activity in Purkinje fibres. J Physiol. 1975 Jul;249(1):40P–41P. [PubMed] [Google Scholar]
- Lederer W. J., Tsien R. W. Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibres. J Physiol. 1976 Dec;263(2):73–100. doi: 10.1113/jphysiol.1976.sp011622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeable junctions. Cold Spring Harb Symp Quant Biol. 1976;40:49–63. doi: 10.1101/sqb.1976.040.01.008. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D., Beeler G. W., Jr Electrophysiologic correlates of the inotropic effects of isoproterenol in canine myocardium. J Mol Cell Cardiol. 1975 Jan;7(1):1–15. doi: 10.1016/0022-2828(75)90002-4. [DOI] [PubMed] [Google Scholar]
- Ochi R. Manganese-dependent propagated action potentials and their depression by electrical stimulation in guinea-pig myocardium perfused by sodium-free media. J Physiol. 1976 Dec;263(2):139–156. doi: 10.1113/jphysiol.1976.sp011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappano A. J., Sperelakis N. Spontaneous contractions of cultured heart cells in high K+ media. Exp Cell Res. 1969 Jan;54(1):58–68. doi: 10.1016/0014-4827(69)90293-6. [DOI] [PubMed] [Google Scholar]
- Peterson O. H., Iwatsuki N. The role of calcium in pancreatic acinar cell stimulus-secretion coupling: an electrophysiological approach. Ann N Y Acad Sci. 1978 Apr 28;307:599–617. doi: 10.1111/j.1749-6632.1978.tb41984.x. [DOI] [PubMed] [Google Scholar]
- REITER M., SCHOEBER H. G. OSCILLATORISCHE KONTRAKTIONSPHAENOMENE DES HERZMUSKELS UNTER DER EINWIRKUNG VON ADRENALIN. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1965 Feb 2;250:21–34. [PubMed] [Google Scholar]
- REITER M. [The origin of "postcontractions" in the myocardium under the influence of calcium and digitalis glycosides in relation to the frequency of stimulation]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1962;242:497–507. [PubMed] [Google Scholar]
- Rang H. P. Acetylcholine receptors. Q Rev Biophys. 1974 Jul;7(3):283–399. doi: 10.1017/s0033583500001463. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P. J., Whittam R. The control by internal calcium of membrane permeability to sodium and potassium. J Physiol. 1971 May;214(3):481–507. doi: 10.1113/jphysiol.1971.sp009445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M. R., Gelband H., Merker C., Hoffman B. F. Mechanisms of digitalis toxicity. Effects of ouabain on phase four of canine Purkinje fiber transmembrane potentials. Circulation. 1973 Apr;47(4):681–689. doi: 10.1161/01.cir.47.4.681. [DOI] [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Cardiac muscle. A comparative study of Purkinje fibers and ventricular fibers. J Cell Biol. 1968 Mar;36(3):497–526. doi: 10.1083/jcb.36.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalle M. Analysis of cardiac pacemaker potential using a "voltage clamp" technique. Am J Physiol. 1966 Jun;210(6):1335–1341. doi: 10.1152/ajplegacy.1966.210.6.1335. [DOI] [PubMed] [Google Scholar]
- Verdonck F., Busselen P., Carmeliet E. Ca-action potentials and contractions of heart muscle in Na-free solutions. Influence of caffeine. Arch Int Physiol Biochim. 1972 Jan;80(1):167–169. [PubMed] [Google Scholar]
- Winegrad S. Resting sarcomere length-tension relation in living frog heart. J Gen Physiol. 1974 Sep;64(3):343–355. doi: 10.1085/jgp.64.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]


