Abstract
1. Electrophysiological properties of soleus motoneurones in adult cats were examined with intracellular electrodes following alterations of activity of the soleus muscle induced by transection of the thoracic spinal cord or by conduction block of the muscle nerve with tetrodotoxin (TTX) cuffs. Attempts were also made to maintain muscle activity by daily stimulation of the maintain muscle activity by daily stimulation of the peripheral nerve. 2. Within 8 days after transection of the thoracic cord, soleus motoneurones showed a significant decrease in the duration of afterhyperpolarization following action potentials. This change in motoneurone properties induced by cord transection was prevented by daily stimulation of the sciatic nerve. 3. Soleus motoneurones showed a significant decrease in the duration of after-hyperpolarization within 8 days after conduction block of the soleus nerve with TTX. This change in montoneurone properties was prevented by daily stimulation of the nerve peripheral to the TTX cuff but not central to the cuff. 4. The soleus muscle showed a significant decrease in weight relative to body weight within 8 days after transection of the thoracic cord. This decrease in muscle weight following cord transection was prevented by daily stimulation of the sciatic nerve. 5. No fibrillation was detected in the soleus muscle 8 days after conduction block of the soleus nerve with TTX. The maximum twitch tension of the soleus muscle evoked by nerve stimulation showed no significant difference between the two sides treated and untreated with TTX. Fast axoplasmic transport measured with cholinesterase as a marker was not affected by TTX. Thus, there was no sign of functional although morphological abnormalities were found in some nerve fibres. 6. It is concluded that motoneurone properties in an adult depend partly upon some factors associated with activity of the innervated muscles and that such trophic signals are retrogradely carried by the motor axons.
Full text
PDF


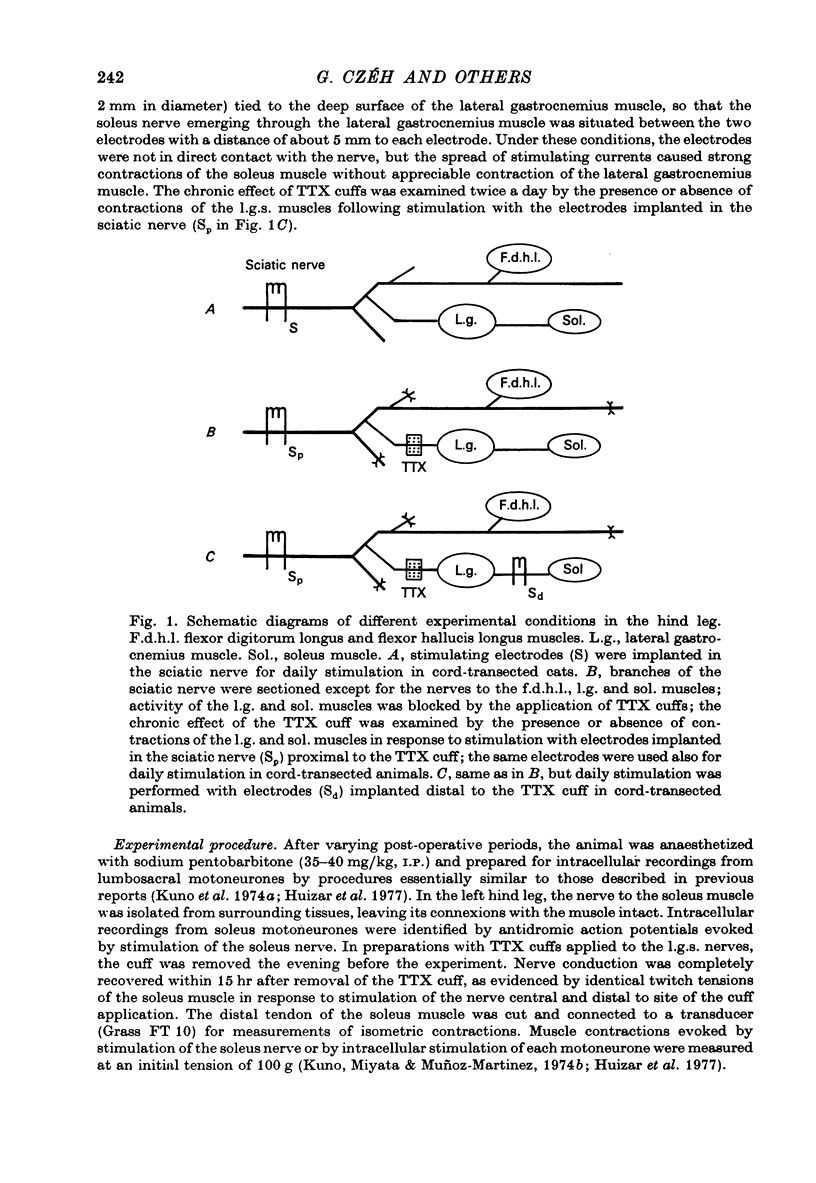
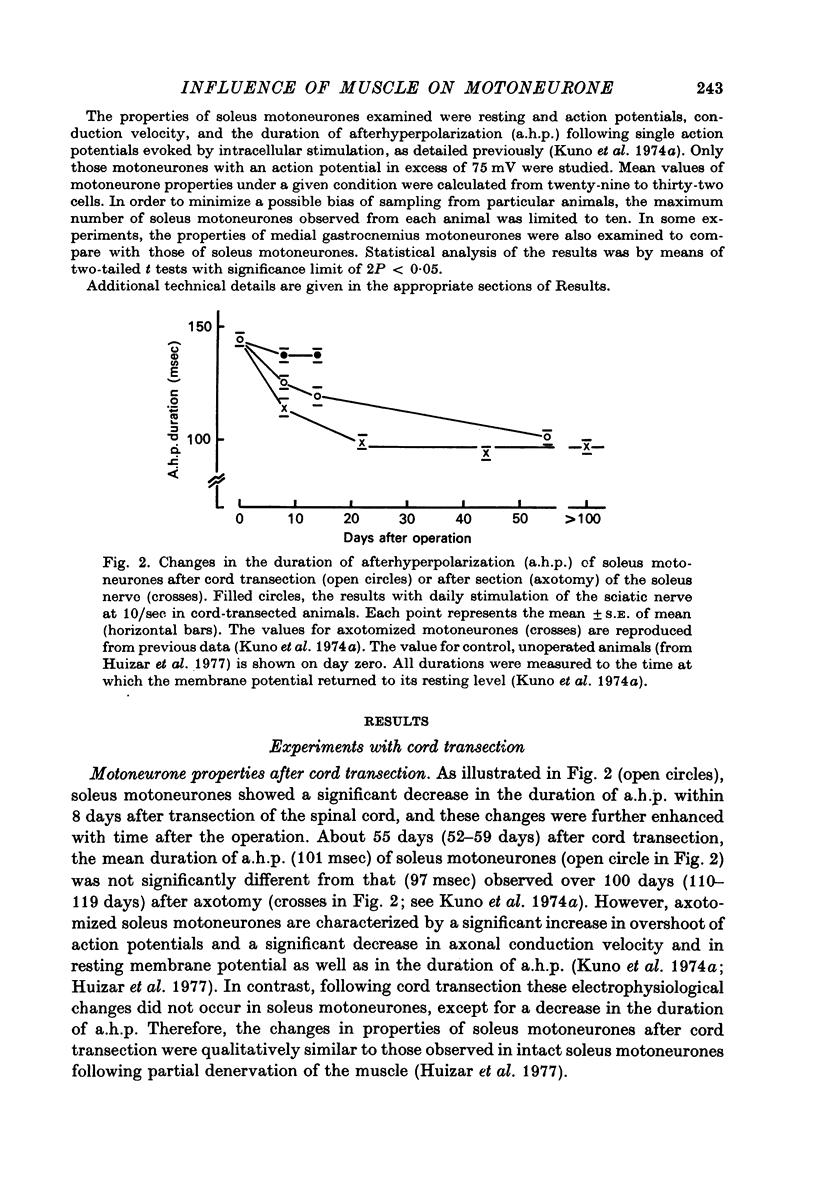
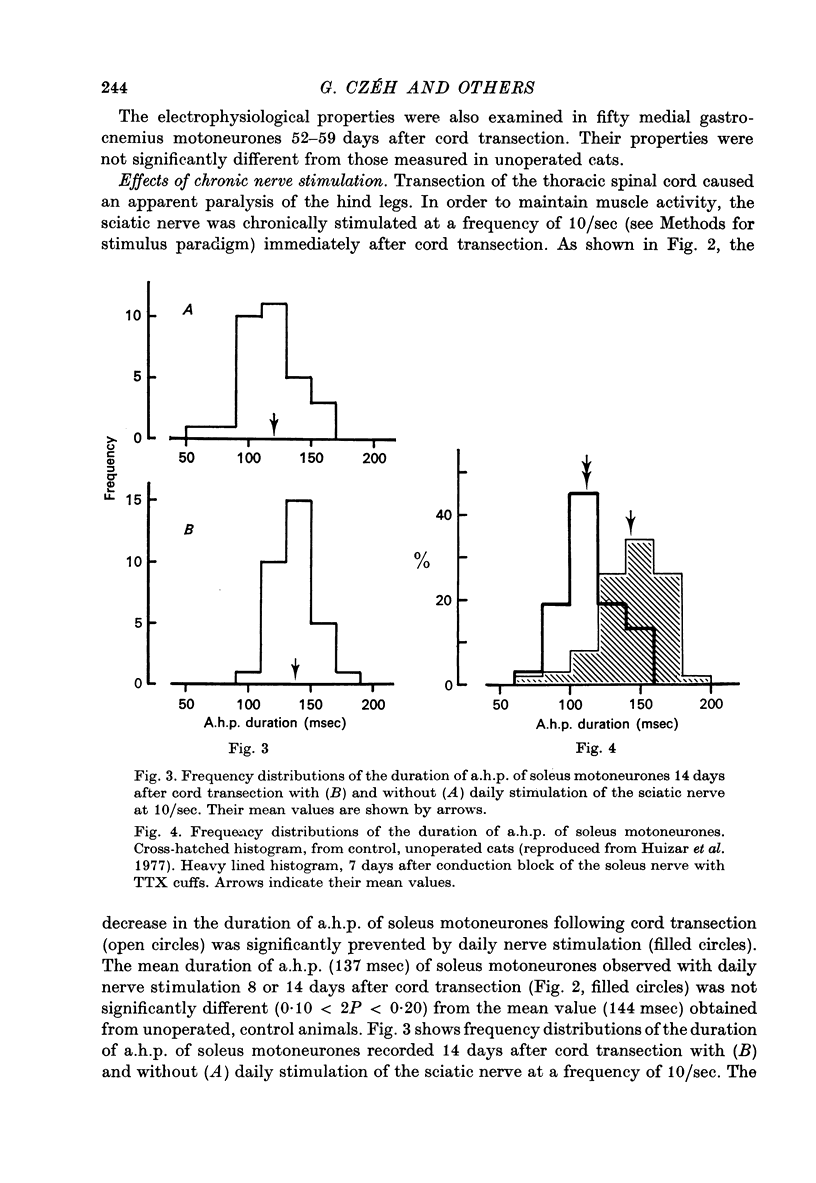

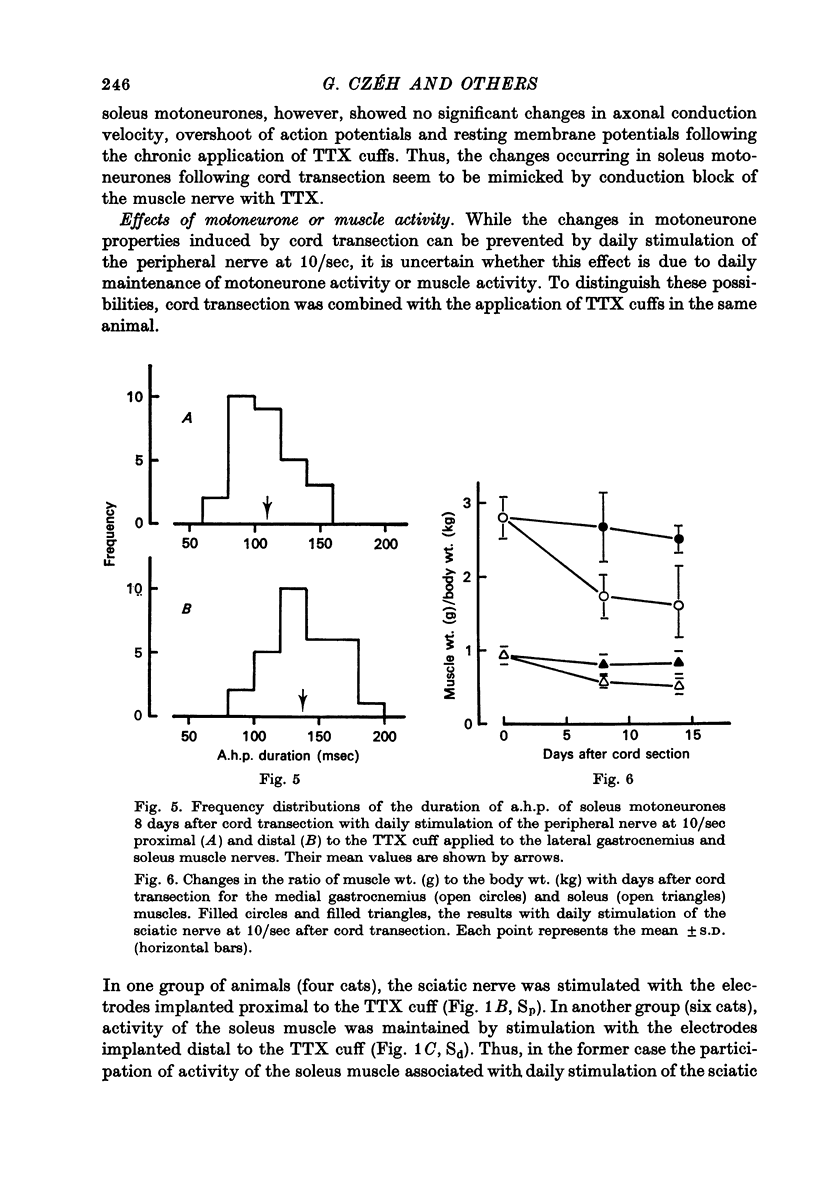






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E., Edström A. Effects of nerve blocking agents on fast axonal transport of proteins in frog sciatic nerves in vitro. Brain Res. 1973 Feb 14;50(1):125–134. doi: 10.1016/0006-8993(73)90599-4. [DOI] [PubMed] [Google Scholar]
- BORISON H. L., McCARTHYLE, CLARK W. G., RADHAKRISHAN N. Vomiting, hypothermia, and respiratory paralysis due to tetrodotoxin (puffer fish poison) in the cat. Toxicol Appl Pharmacol. 1963 May;5:350–357. doi: 10.1016/0041-008x(63)90094-2. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Goodwin G. M., Ironton R. Prevention of motor nerve sprouting in botulinum toxin poisoned mouse soleus muscles by direct stimulation of the muscle [proceedings]. J Physiol. 1977 May;267(1):42P–43P. [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Motor neurone sprouting induced by prolonged tetrodotoxin block of nerve action potentials. Nature. 1977 Feb 3;265(5593):459–461. doi: 10.1038/265459a0. [DOI] [PubMed] [Google Scholar]
- DeVilliers R., Nosé Y., Meier W., Kantrowitz A. Long-term, continuous electrostimulation of a peripheral nerve. Trans Am Soc Artif Intern Organs. 1964;10:357–365. [PubMed] [Google Scholar]
- Duchen L. W., Strich S. J. The effects of botulinum toxin on the pattern of innervation of skeletal muscle in the mouse. Q J Exp Physiol Cogn Med Sci. 1968 Jan;53(1):84–89. doi: 10.1113/expphysiol.1968.sp001948. [DOI] [PubMed] [Google Scholar]
- EDDS M. V., Jr Collateral nerve regeneration. Q Rev Biol. 1953 Sep;28(3):260–276. doi: 10.1086/399699. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fernandez H. L., Ramirez B. U. Muscle fibrillation induced by blockage of axoplasmic transport in motor nerves. Brain Res. 1974 Oct 25;79(3):385–395. doi: 10.1016/0006-8993(74)90436-3. [DOI] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D., STEG G. Tonic and phasic ventral horn cells differentiated by post-tetanic potentiation in cat extensors. Acta Physiol Scand. 1956 Sep 26;37(2-3):114–126. doi: 10.1111/j.1748-1716.1956.tb01347.x. [DOI] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G., SKOGLUND S., STEG G. Differentiation of tonic from phasic alpha ventral horn cells by stretch, pinna and crossed extensor reflexes. J Neurophysiol. 1957 Sep;20(5):470–481. doi: 10.1152/jn.1957.20.5.470. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F. The influence of activity on muscle size and protein turnover. J Physiol. 1977 Jan;264(1):283–296. doi: 10.1113/jphysiol.1977.sp011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol. 1977 Jan;264(1):267–282. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBURGER V. Regression versus peripheral control of differentiation in motor hypoplasia. Am J Anat. 1958 May;102(3):365–409. doi: 10.1002/aja.1001020303. [DOI] [PubMed] [Google Scholar]
- HUGHES A. An experimental study on the relationships between limb and spinal cord in the embryo of Eleutherodactylus martinicensis. J Embryol Exp Morphol. 1962 Dec;10:575–601. [PubMed] [Google Scholar]
- HUGHES A. Cell degeneration in the larval ventral horn of Xenopus laevis (Daudin). J Embryol Exp Morphol. 1961 Jun;9:269–284. [PubMed] [Google Scholar]
- Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. J Comp Neurol. 1975 Apr 15;160(4):535–546. doi: 10.1002/cne.901600408. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyday M., Hamburger V. Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J Comp Neurol. 1976 Dec 1;170(3):311–320. doi: 10.1002/cne.901700304. [DOI] [PubMed] [Google Scholar]
- Holtzman E., Freeman A. R., Kashner L. A. Stimulation-dependent alterations in peroxidase uptake at lobster neuromuscular junctions. Science. 1971 Aug 20;173(3998):733–736. doi: 10.1126/science.173.3998.733. [DOI] [PubMed] [Google Scholar]
- Huizar P., Kuno M., Kudo N., Miyata Y. Reaction of intact spinal motoneurones to partial denervation of the muscle. J Physiol. 1977 Feb;265(1):175–191. doi: 10.1113/jphysiol.1977.sp011711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpati G., Engel W. K. Correlative histochemical study of skeletal muscle after suprasegmental denervation, peripheral nerve section, and skeletal fixation. Neurology. 1968 Jul;18(7):681–692. doi: 10.1212/wnl.18.7.681. [DOI] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Differential reaction of fast and slow alpha-motoneurones to axotomy. J Physiol. 1974 Aug;240(3):725–739. doi: 10.1113/jphysiol.1974.sp010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Properties of fast and slow alpha motoneurones following motor reinnervation. J Physiol. 1974 Oct;242(1):273–288. doi: 10.1113/jphysiol.1974.sp010706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie P. A., Collier B., Tenehouse A. Comparison of alpha-bungarotoxin binding to skeletal muscles after inactivity or denervation. Nature. 1976 Mar 25;260(5549):349–350. doi: 10.1038/260349a0. [DOI] [PubMed] [Google Scholar]
- Lavoie P. A., Collier B., Tenenhouse A. Role of skeletal muscle activity in the control of muscle acetylcholine sensitivity. Exp Neurol. 1977 Jan;54(1):148–171. doi: 10.1016/0014-4886(77)90242-4. [DOI] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H., Dahl H. A. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proc R Soc Lond B Biol Sci. 1974 Aug 27;187(1086):99–103. doi: 10.1098/rspb.1974.0064. [DOI] [PubMed] [Google Scholar]
- Ochs S., Hollingsworth D. Dependence of fast axoplasmic transport in nerve on oxidative metabolism. J Neurochem. 1971 Jan;18(1):107–114. doi: 10.1111/j.1471-4159.1971.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B., Griffin J. W. Effect of muscle disuse on acetylcholine receptors. Nature. 1976 Mar 25;260(5549):352–353. doi: 10.1038/260352a0. [DOI] [PubMed] [Google Scholar]
- Pilar G., Landmesser L. Ultrastructural differences during embryonic cell death in normal and peripherally deprived ciliary ganglia. J Cell Biol. 1976 Feb;68(2):339–356. doi: 10.1083/jcb.68.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestige M. C. Evidence that at least some of the motor nerve cells that die during development have first made peripheral connections. J Comp Neurol. 1976 Nov 1;170(1):123–133. doi: 10.1002/cne.901700109. [DOI] [PubMed] [Google Scholar]
- Prestige M. C. The control of cell number in the lumbar ventral horns during the development of Xenopus laevis tadpoles. J Embryol Exp Morphol. 1967 Dec;18(3):359–387. [PubMed] [Google Scholar]
- Salmons S., Vrbová G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol. 1969 May;201(3):535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. Some metabolic responses of axotomized neurones to contact between their axons and denervated muscle. J Physiol. 1970 Sep;210(2):321–343. doi: 10.1113/jphysiol.1970.sp009213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. Some responses of neurones of dorsal root ganglia to axotomy. J Physiol. 1973 May;231(1):41P–42P. [PubMed] [Google Scholar]
- Watson W. E. The response of motor neurones to intramuscular injection of botulinum toxin. J Physiol. 1969 Jun;202(3):611–630. doi: 10.1113/jphysiol.1969.sp008830. [DOI] [PMC free article] [PubMed] [Google Scholar]


