Abstract
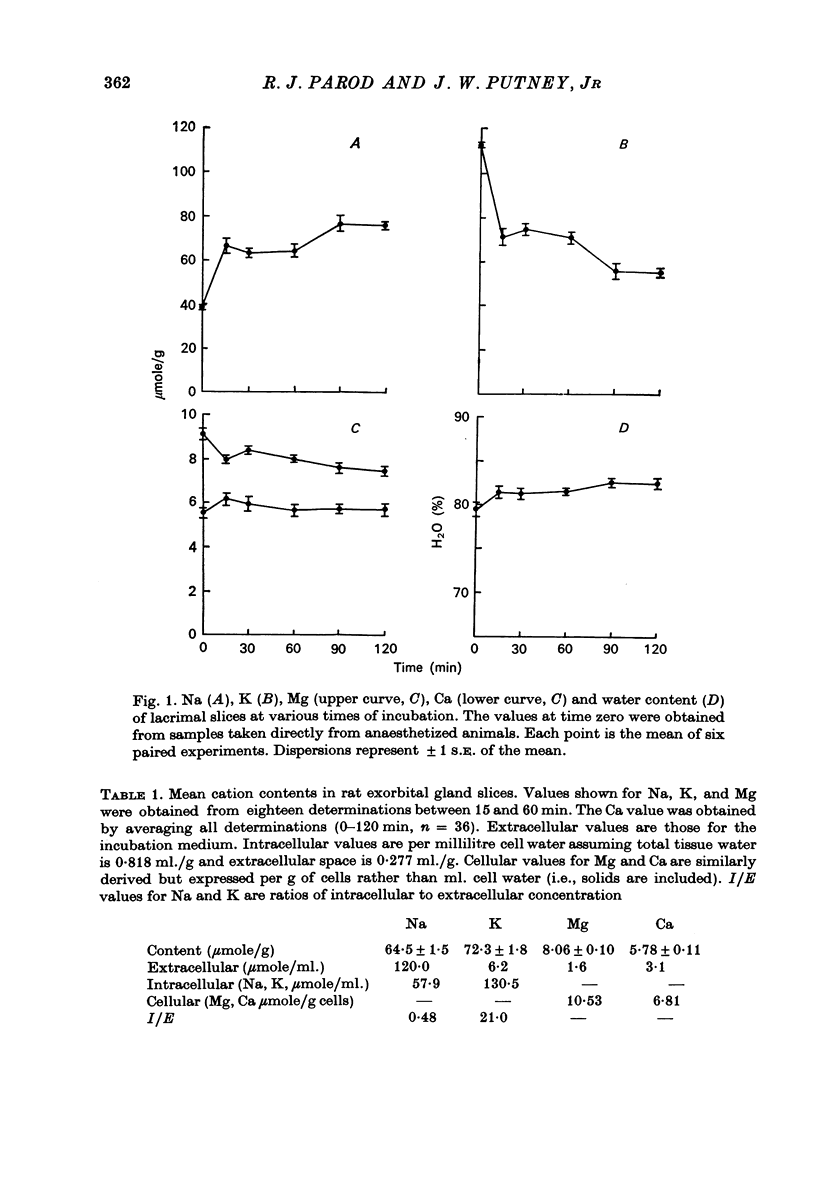
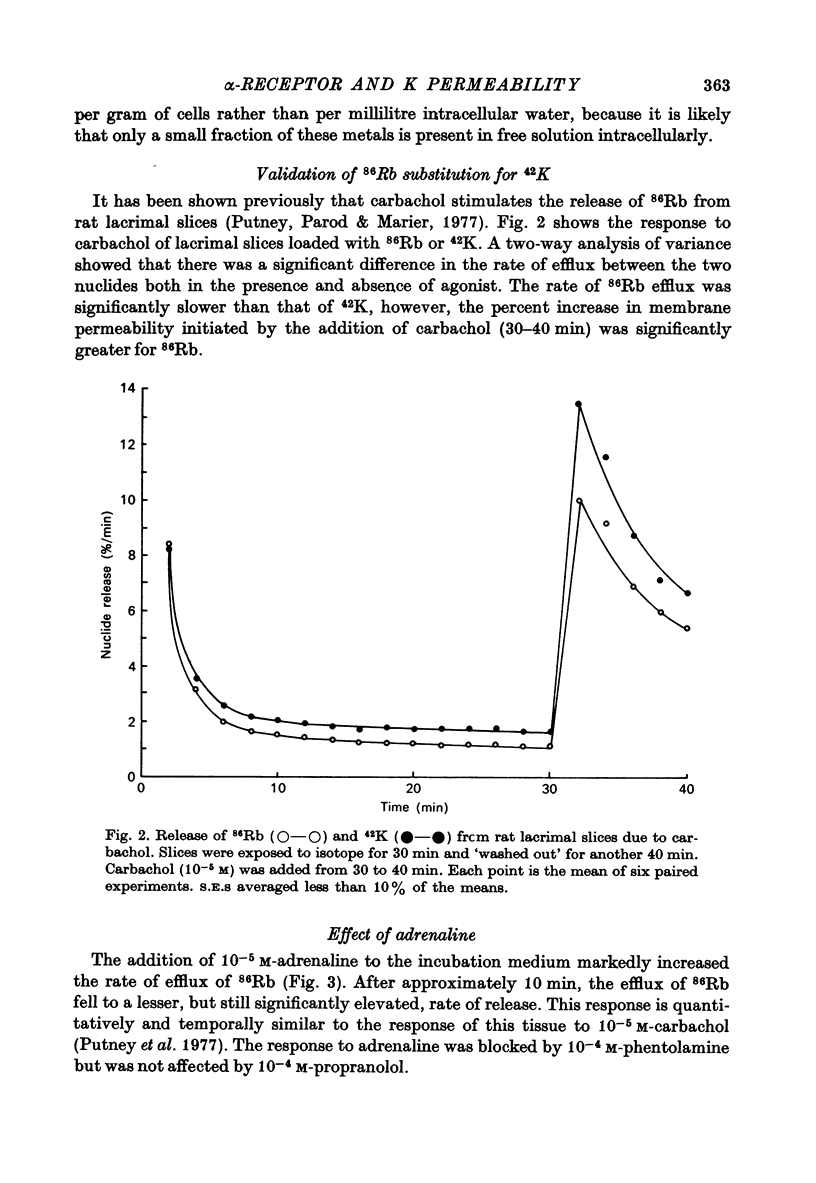
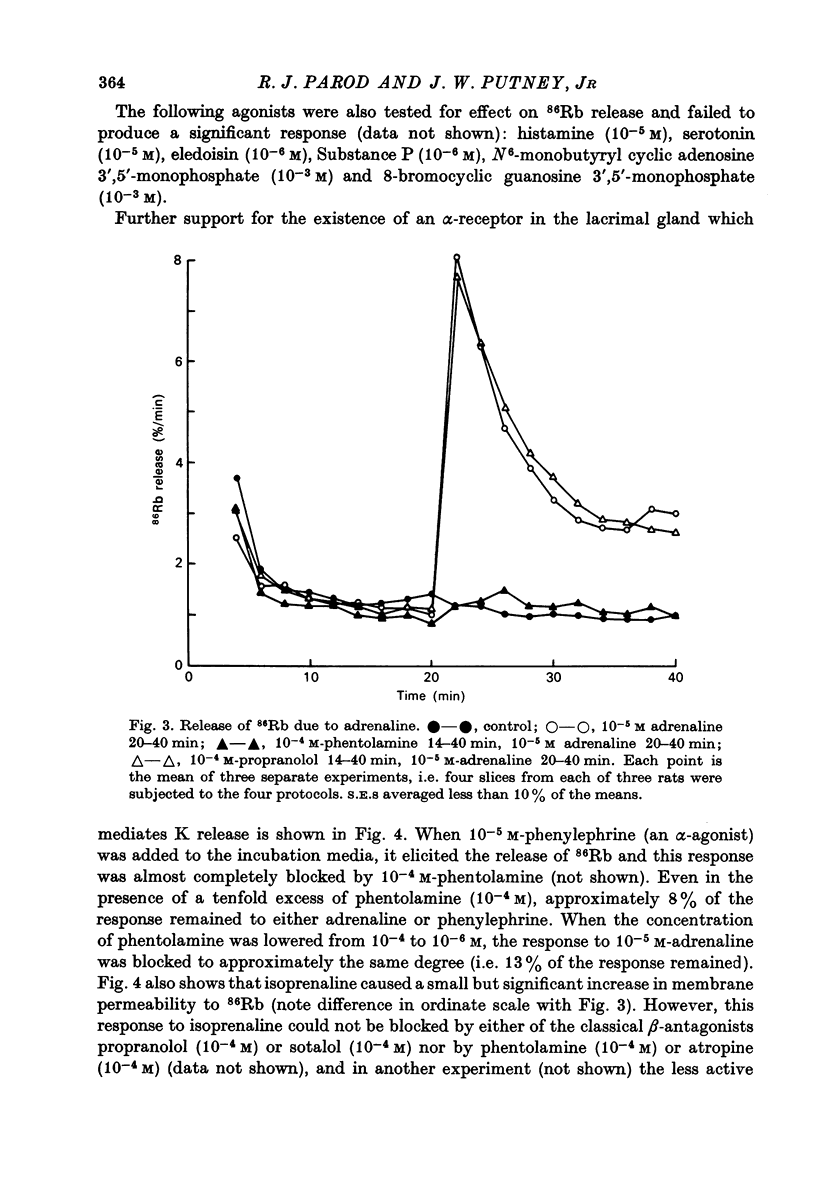
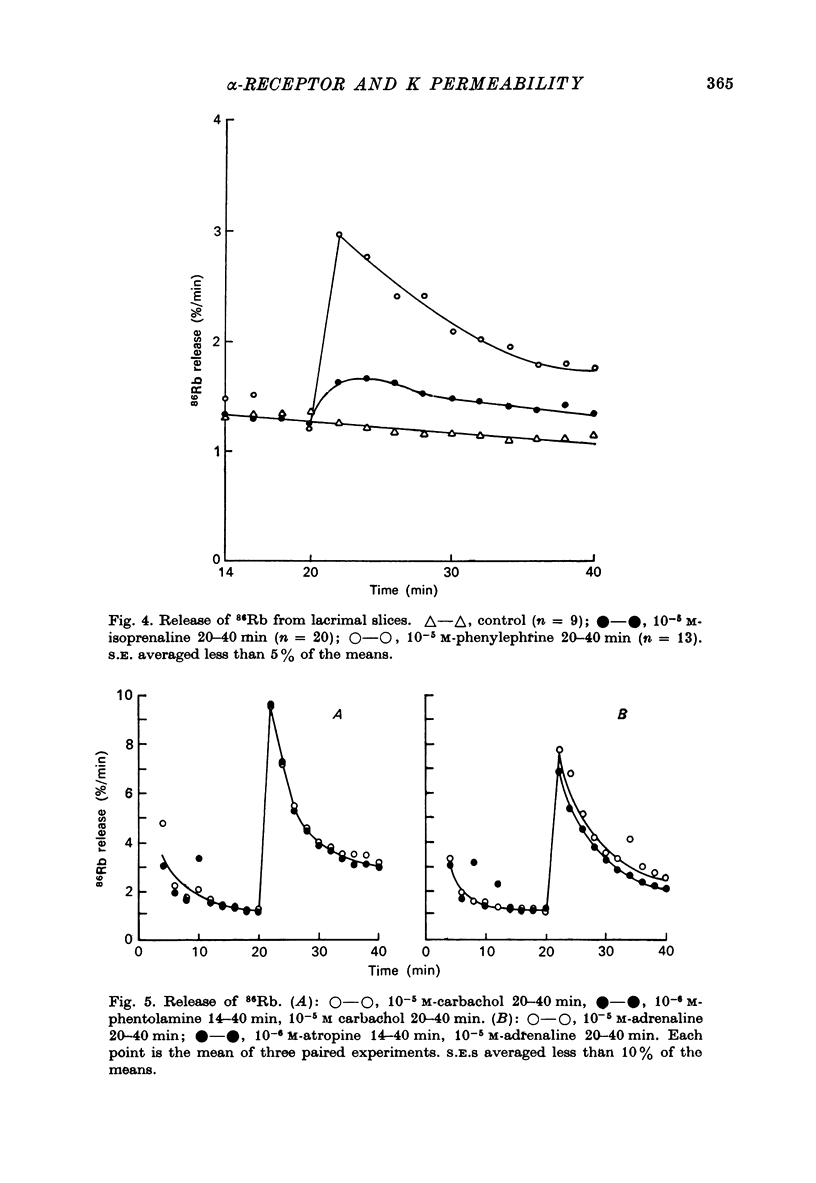
1. Rat lacrimal gland slices, incubated in a balanced, buffered salt solution, were found to be physiologically stable for up to 2 hr with respect to O2 consumption, extracellular space, and water and ion content. 2. The release of 86Rb serves as a good substitute for 42K in monitoring the movement of K through the cell membrane. 3. Adrenaline appears to increase membrane permeability to K as evidenced by an increase in the rate of 86Rb efflux. 4. This response to adrenaline was blocked by phentolamine but not by propranolol and was mimicked by phenylephrine but not by isoprenaline. 5. The magnitude of the 86Rb release indicates that it is being released, at least in part, from the lacrimal gland acinar cell. 6. It is concluded that the lacrimal gland acinar cell has an alpha-adrenergic receptor, activation of which leads to an increase in membrane permeability to K.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOTELHO S. Y. TEARS AND THE LACRIMAL GLAND. Sci Am. 1964 Oct;211:78–86. doi: 10.1038/scientificamerican1064-78. [DOI] [PubMed] [Google Scholar]
- Batzri S., Selinger Z., Schramm M., Robinovitch M. R. Potassium release mediated by the epinephrine -receptor in rat parotid slices. Properties and relation to enzyme secretion. J Biol Chem. 1973 Jan 10;248(1):361–368. [PubMed] [Google Scholar]
- Botelho S. Y., Goldstein A. M., Martinez E. V. Norepinephrine-responsive beta-adrenergic receptors in rabbit lacrimal gland. Am J Physiol. 1973 May;224(5):1119–1122. doi: 10.1152/ajplegacy.1973.224.5.1119. [DOI] [PubMed] [Google Scholar]
- Botelho S. Y., Martinez E. V., Pholpramool C., Prooyen H. C., Janssen J. T., De Palau A. Modification of stimulated lacrimal gland flow by sympathetic nerve impulses in rabbit. Am J Physiol. 1976 Jan;230(1):80–84. doi: 10.1152/ajplegacy.1976.230.1.80. [DOI] [PubMed] [Google Scholar]
- Goldstein A. M., De Palau A., Botelho S. Y. Inhibition and facilitation of pilocarpine-induced lacrimal flow by norepinephrine. Invest Ophthalmol. 1967 Oct;6(5):498–511. [PubMed] [Google Scholar]
- Herzog V., Miller F. Endogenous peroxidase in the lacrimal gland of the rat and its differentiation against injected catalase and horseradish-peroxidase. Histochemie. 1972;30(3):235–246. doi: 10.1007/BF00277594. [DOI] [PubMed] [Google Scholar]
- Herzog V., Sies H., Miller F. Exocytosis in secretory cells of rat lacrimal gland. Peroxidase release from lobules and isolated cells upon cholinergic stimulation. J Cell Biol. 1976 Sep;70(3):692–706. doi: 10.1083/jcb.70.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilden S., Hokin L. E. Active potassium transport coupled to active sodium transport in vesicles reconstituted from purified sodium and potassium ion-activated adenosine triphosphatase from the rectal gland of Squalus acanthias. J Biol Chem. 1975 Aug 25;250(16):6296–6303. [PubMed] [Google Scholar]
- Leslie B. A., Putney J. W., Jr, Sherman J. M. alpha-Adrenergic, beta-adrenergic and cholinergic mechanisms for amylase secretion by rat parotid gland in vitro. J Physiol. 1976 Sep;260(2):351–370. doi: 10.1113/jphysiol.1976.sp011519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J. R., Quissell D. O., Giles M. Potassium release from the rat submaxillary gland in vitro. I. Induction by catecholamines. J Pharmacol Exp Ther. 1976 Aug;198(2):385–394. [PubMed] [Google Scholar]
- Petersen O. H., Gray T. A., Hall R. A. The relationship between stimulation-induced potassium release and amylase secretion in the mouse parotid. Pflugers Arch. 1977 Jul 19;369(3):207–211. doi: 10.1007/BF00582186. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Biphasic modulation of potassium release in rat parotid gland by carbachol and phenylephrine. J Pharmacol Exp Ther. 1976 Aug;198(2):375–384. [PubMed] [Google Scholar]
- Putney J. W., Jr, Parod R. J., Marier S. H. Control by calcium of protein discharge and membrane permeability to potassium in the rat lacrimal gland. Life Sci. 1977 Jun 1;20(11):1905–1911. doi: 10.1016/0024-3205(77)90227-2. [DOI] [PubMed] [Google Scholar]
- Ruskell G. L. Changes in nerve terminals and acini of the lacrimal gland and changes in secretion induced by autonomic denervation. Z Zellforsch Mikrosk Anat. 1969;94(2):261–281. doi: 10.1007/BF00339361. [DOI] [PubMed] [Google Scholar]
- Ruskell G. L. Nerve terminals and epithelial cell variety in the human lacrimal gland. Cell Tissue Res. 1975;158(1):121–136. doi: 10.1007/BF00219955. [DOI] [PubMed] [Google Scholar]
- Schramm M. Amylase secretion in rat parotid slices by apparent activation of endogenous catecholamine. Biochim Biophys Acta. 1968 Oct 15;165(3):546–549. doi: 10.1016/0304-4165(68)90238-9. [DOI] [PubMed] [Google Scholar]


