Abstract
Extracellular nucleotides are signaling molecules whose receptor-mediated effects are involved in a variety of physiological responses in mammalian tissues. An overwhelming body of data indicate that inflammatory and other immune responses can be modulated by the availability and local concentrations of nucleotides via nucleotide receptor signaling, but this is only just beginning to be investigated in the context of infectious disease. Evidence is provided here that the parasitic nematode Trichinella spiralis can catalyze the conversion and thus modulate both the availability and concentration of extracellular nucleotides by means of the following secreted exoenzymes: apyrase, 5′-nucleotidase, and adenosine deaminase. These enzymes were characterized in terms of substrate specificity, kinetic behavior, pH, divalent cation preferences, and response to a series of compounds. The secreted 5′-nucleotidase was identified as a protein with an apparent molecular mass of 67 kDa after N-terminal amino acid sequencing of the purified protein. The presence of adenosine deaminase was confirmed in the secreted products by Western blotting with an antibody against a mammalian enzyme, as a protein with an apparent molecular mass of 38 kDa. These secreted proteins constitute an enzymatic cascade which catalyzes the degradation of extracellular nucleotides, with a potential physiological role in the regulation of purinergic signaling.
Purinergic signaling relies on interactions between extracellular nucleotides and plasma membrane-bound receptors. This type of signaling therefore depends on nucleotide release, metabolism by enzymes acting extracellularly, and the presence of receptors which will selectively bind the resulting nucleotides and transduce the signal to the interior of the cell.
Receptors for purine and pyrimidine nucleotides exhibit a wide tissue distribution including all hematopoietic cells (1, 16, 29, 48), and numerous studies have demonstrated that extracellular nucleotides regulate a broad range of inflammatory and other immune responses (14, 15, 17, 39, 47). There is now clear evidence that nucleotides are released extracellularly in a regulated manner. In addition, large amounts of nucleotides are released upon mechanical stimulation (such as stretching) of different cell types, including epithelial and endothelial cells, and massive release of nucleotides into the extracellular space follows cell damage resulting in the activation of purinergic receptors (34, 36, 37, 39, 65). The latter are broadly divided into two categories, the P1 and the P2 receptors. P1 receptors respond specifically to adenosine (and some to inosine), whereas P2 receptors are responsive to ATP, UTP, ADP, and UDP (14, 48, 39). P1 receptors have been classified on the basis of biochemical and pharmacological properties into four G-protein coupled receptor subclasses, designated A1, A2A, A2B and A3. P2 receptors are subdivided into the G-protein coupled P2Y receptors and the ligand-gated ion channel P2X receptors. To date, seven mammalian P2X receptors (P2X1-7) and seven P2Y (P2Y1,2,4,6,11,12,13) receptors have been cloned (1, 9, 10, 16, 48).
The concentration of extracellular nucleotides, and the cellular responses to these molecules, is regulated by the action of a ubiquitous family of membrane-bound nucleotide-metabolizing enzymes, such as ecto-ATP/ADPases and ecto-nucleotidases. Ecto-protein kinases and ecto-nucleoside diphosphate kinases (NDPKs) also modulate the availability of different nucleotides and are of physiological importance in determining the activation of various purinergic receptor subtypes and the nucleotide-mediated signaling events (35, 37, 64). A large body of data indicate that inflammatory responses are regulated by the availability and/or local concentrations of nucleotides via purine/pyrimidine receptor signaling, which affects events such as cytokine release, mast cell degranulation, expression of adhesion molecules on the endothelium, and platelet aggregation (16, 27, 30, 39, 47).
Trichinella spiralis is a ubiquitous nematode parasite of a wide variety of mammalian species, including humans. T. spiralis infective larvae invade the small intestine by migration through the mucosal epithelial cells (13). The effector mechanisms which result in parasite expulsion are not fully understood, but it is a generally held opinion that this is effected by a combination of alterations in intestinal physiology and Th2-mediated inflammatory responses (18, 21). Infective larvae possess a large secretory organelle termed the stichosome, the source of secreted proteins which can be recovered from in vitro culture of parasites. It is considered likely that these secreted products are involved in the survival and development of parasites within host cells (13).
As infective larvae of T. spiralis cause extensive tissue damage during migration through the intestinal epithelium, high concentrations of nucleotides must be liberated with profound effects on induction of local inflammation. We have previously demonstrated that T. spiralis infective larvae possess membrane-bound ecto-protein kinase and phosphatase activities and secrete serine/threonine protein kinases as well as a nucleoside diphosphate kinase (3, 20, 53). It is reported here that infective larvae additionally secrete a repertoire of proteins which constitute an enzymatic cascade of nucleotide-metabolizing molecules, which would directly affect the availability and local concentrations of extracellular nucleotides. It is postulated that these enzymes could potentially regulate purine/pyrimidine receptor activation and the ensuing inflammatory response.
MATERIALS AND METHODS
Parasites.
Infective larvae of Trichinella spiralis were recovered from outbred rats 2 months after oral infection as previously described (3). Parasites were cultured in serum-free RPMI-1640 containing 0.25% glucose, 2 mM l-glutamine, 100 U of penicillin ml−1, 100 μg of streptomycin ml−1, and 20 μg of gentamicin ml−1 at 37°C, 5% CO2 for 72 h with a daily change of medium. Secreted products were pooled, cleared through 0.2-μm-pore-size filters, dialyzed against 25 mM HEPES (pH 7.5), and concentrated by passage through Centricon 10 microconcentrators (Amicon). Protein content was determined by the BCA microplate assay (Pierce).
Enzyme assays.
Enzymatic phosphate hydrolysis was followed by measuring the release of inorganic phosphate from the appropriate substrates by the malachite green-phosphomolybdate reagent (4, 28). Released inorganic phosphate was measured by determining the absorbance at 660 nm using a microtiter plate reader (Spectra Max 340PC; Molecular Devices) and quantified by comparison to a standard curve for inorganic phosphate obtained with sodium dihydrogen phosphate. Reactions were carried out in a buffer containing 25 mM HEPES (pH 7.5), 150 mM NaCl, 5 mM MgCl2, and 2.5 mM dithiothreitol (DTT), unless otherwise stated, and initiated by the addition of appropriate concentrations of secreted proteins and nucleotide substrates. The plates were incubated at 37°C for 10 min (during which time the reaction was found to be linear) prior to the addition of the chromogenic reagent. Controls for nonenzymatic hydrolysis of phosphate were always carried out in parallel. Determination of kinetic parameters for apyrase and 5′-nucleotidase were performed in the above buffer in the presence of various concentrations of UDP or AMP, respectively. Curve fitting to the data points was performed by GraphPad Prism software.
Adenosine deaminase activity in the secreted products of T. spiralis was measured in quartz cuvettes using a buffer containing 25 mM HEPES (pH 7.5) and 150 mM NaCl, unless otherwise stated. The reactions were initiated by the addition of the appropriate amounts of adenosine. Either samples were scanned at defined time intervals from 230 to 320 nm or the absorbance was measured at 265 nm, using a Hewlett Packard 8453 spectrophotometer with the cuvettes maintained at 37°C. One unit of adenosine deaminase activity is defined as the amount of enzyme required to catalyze the deamination of 1 μmol of adenosine per min (ΔA = 8.6 min−1 ml−1 at 265 nm) (2).
Protein purification, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), N-terminal sequencing, and Western blotting.
The 5′-nucleotidase was purified from total parasite-secreted proteins by ATP-affinity chromatography following the procedure previously described (20). Following adsorption of secreted products and washing with KCl, the protein was eluted from the affinity matrix by 2 mM ATP. Protein samples were resolved by SDS-12% PAGE. For sequencing, protein samples were transferred onto Sequi-Blot polyvinylidene difluoride membranes (Bio-Rad) following the manufacturer's instructions, and N-terminal sequencing was carried out by Severn Biotech Ltd. (Worcestershire, United Kingdom). For Western blotting the proteins were transferred onto nitrocellulose membranes and overlaid with a 1:1,000 dilution of a rabbit antibody to bovine adenosine deaminase (Alpha Diagnostic International, San Antonio, Tex.). Binding was determined by standard procedures utilizing horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (Amersham RPN 2209).
RESULTS
Infective larvae of T. spiralis migrate through intestinal epithelial cells causing extensive tissue damage, which would inevitably result in the release of high concentrations of nucleotides. The secreted products of T. spiralis were therefore examined for enzymatic activities characteristic of proteins involved in the catabolism of extracellular adenine nucleotides, and the data obtained are shown in Table 1. Enzymes which catalyzed the hydrolysis of ADP and AMP were clearly present. Interestingly, only minimal activity towards magnesium ATP (but not sodium ATP) was detected under the conditions of the experiments. All other available nucleoside 5′-triphosphates were used as substrates, i.e., UTP, CTP, GTP, ITP, and TTP, but no measurable activity was detected (data not shown). The secreted products of T. spiralis larvae thus exhibit an apyrase (nucleoside 5′-diphosphate phosphohydrolase) and 5′-nucleotidase activities, but no significant activity directed against nucleoside 5′-triphosphates. The assay used measures release of inorganic phosphate, and thus, the apyrase activity reported here is an overestimate, since AMP, the product of this enzyme's action, would subsequently be hydrolyzed by the 5′- nucleotidase. Nevertheless, the 5′-nucleotidase exhibited a high specific activity. The possibility that inorganic pyrophosphatase or other nonspecific phosphatases were present in the secreted products was investigated, but no activity was detected when sodium pyrophosphate (at 2 or 5 mM) or phosphate esters such as glucose-6-phosphate (5 mM) and β-glycerophosphate (5 mM) were used (data not shown).
TABLE 1.
Enzymatic activities in T. spiralis secreted proteins involved in the extracellular metabolism of nucleotidesa
| Activity | Substrate | Sp act (nmol/min/mg of total secreted protein) |
|---|---|---|
| ATPase | (Mg)ATP | 105 ± 17 (6) |
| (Na)ATP | ND (6) | |
| ADPase | ADP | 1,230 ± 37 (15) |
| Nucleotidase | 5′-AMP | 4,960 ± 98 (15) |
| 3′-AMP | ND (5) |
Samples were assayed in triplicate, and the results are expressed as means ± standard deviations for the number of preparations tested, shown in parentheses. Substrates were used at 2 mM. ND, (not detected).
The substrate specificities of the apyrase and the 5′-nucleotidase were examined, and the data obtained are shown in Fig. 1. The 5′-nucleotidase is virtually monospecific for AMP, since all other nucleoside monophosphates tested were poor substrates (Fig. 1A). It was surprising, however, to observe that the apyrase showed a distinct preference for UDP, with minor activities measurable for all other nucleoside diphosphates (Fig. 1B). The specific activity of the enzyme with UDP as the substrate was estimated to be 2,120 ± 52 nmol/min/mg of total secreted protein.
FIG. 1.
Substrate specificity of the 5′-nucleotidase (A) and the apyrase (B) in T. spiralis secreted products. The data are expressed as a percentage of the maximal activities obtained and are the means for five independent experiments carried out in triplicate.
The 5′-nucleotidase and the apyrase activities were further characterized, using UDP as the substrate for the latter enzyme, and the data are shown in Fig. 2. In the absence of divalent cations, significant 5′-nucleotidase activity but minimal apyrase activity were observed. Magnesium ions were activators of both enzymes, although the activity of the 5′-nucleotidase was not absolutely dependent on the presence of the cation. Calcium ions could not substitute for magnesium and indeed inhibited apyrase activity. Zinc was also inhibitory for both enzymatic activities. Addition of the reductant DTT had a profound effect on apyrase activity and induced an additional 10% to 20% increase in 5′-nucleotidase activity. This would suggest the involvement of thiol groups in the active site of at least the former enzyme. Both enzymes were active in the alkaline pH region, exhibiting a broad optimum between the values of 7.0 and 8.5. A similar pH profile and similar ion and reductant requirements were obtained when ADP instead of UDP was used as the substrate for the apyrase (data not shown).
FIG. 2.
Ion and reductant requirements for maximal activities for the 5′-nucleotidase (white bars) and apyrase (black bars) in T. spiralis secreted products. Measurements were performed in 25 mM HEPES (pH 7.5), 150 mM NaCl (column 1) with the following additions: column 2, 5 mM MgCl2; column 3, 5 mM CaCl2; column 4, 5 mM ZnCl2; column 5, 5 mM MgCl2 plus 2.5 mM DTT. The data are expressed as a percentage of the maximal activities obtained for each enzyme and are the means for five independent experiments carried out in triplicate.
Sensitivity to various compounds was tested, and the data are shown in Table 2. Levamisole had no significant effect on the enzymes, thus confirming the absence of an alkaline phosphatase in the secreted products. Suramin, a purinergic receptor antagonist, inhibited both enzymes but had a more pronounced effect on the 5′-nucleotidase activity. Sodium azide, which has been used to differentiate apyrases from other nucleotide-metabolizing enzymes (8), had no significant effect on the activities of the enzymes studied here. N-ethylmaleimide inhibited both enzymes, in particular apyrase activity, reinforcing the suggestion that thiol groups are involved in catalysis. Fluoride was inhibitory to both enzymes. Adenosine 5′-[α,β-methylene]diphosphate (p[CH2]pA) and concanavalin A have been used as specific inhibitors of ecto-5′-nucleotidases (63). As seen in Table 2, p[CH2]pA indeed inhibited the secreted 5′-nucleotidase activity, but it also had an inhibitory effect on the apyrase. Concanavalin A at concentrations below 50 μg/ml had no significant effect on either of the enzymatic activities but exhibited some inhibition of the 5′-nucleotidase activity at 100 μg/ml.
TABLE 2.
Effect of various compounds on 5′-nucleotidase and apyrase activitiesa
| Addition | Relative activity (%)
|
|
|---|---|---|
| 5′-nucleotidase | Apyrase | |
| None | 100 | 100 |
| NaN3 (5 mM) | 92 ± 5 | 84 ± 4 |
| N-ethylmaleimide (5 mM) | 68 ± 5 | 23 ± 8 |
| NaF (10 mM) | 10 ± 3 | 7 ± 2 |
| p[CH2]pA (250 μM) | 30 ± 8 | 40 ± 5 |
| Suramin (5 mM) | 15 ± 6 | 64 ± 7 |
| Levamisole (1 mM) | 99 ± 3 | 91 ± 6 |
| Concanavalin A (100 μg/ml) | 75 ± 8 | 98 ± 9 |
Samples were assayed in triplicate, and results are expressed as the means ± standard deviations for five experiments performed using different preparations. The apyrase activity was assayed with UDP as the substrate.
Kinetic parameters for the enzymes were derived, and these are shown in Fig. 3 for the 5′-nucleotidase and Fig. 4 for the apyrase. The rate of phosphate release was found to be linear within the first 15 min of the reaction for both enzymes. Initial velocities were plotted against AMP concentration for the 5′-nucleotidase and correspond to sigmoidal kinetics (Fig. 3). Curve fitting of the data points to the Hill equation resulted in a Vmax of 2.517 ± 0.096 nmol/min, substrate concentration at half-maximal velocity of 0.287 mM ± 0.02, and a Hill coefficient of 2.31. Similarly, the kinetic parameters for UDP hydrolysis by the apyrase were examined. Initial velocities plotted over a range of UDP concentrations again followed sigmoidal kinetics (Fig. 4), and curve fitting of the data to the Hill equation resulted in a Vmax of 1.059 ± 0.042 nmol/min, substrate concentration at half-maximal velocity of 0.102 mM ± 0.03, and a Hill coefficient of 1.53.
FIG. 3.
Substrate concentration dependence of the rate of AMP hydrolysis by the 5′-nucleotidase in T. spiralis secreted products. The values are averages for five experiments performed in triplicate. The data were fitted to the Hill equation, and curve fitting of the data resulted in an r2 value of 0.998. The calculated kinetic parameters are described in the text.
FIG. 4.
Substrate concentration dependence of the rate of UDP hydrolysis by the apyrase in T. spiralis secreted products. The values are averages for five experiments performed in triplicate. The data were fitted to the Hill equation, and curve fitting of the data resulted in an r2 value of 0.995. Kinetic parameters are described in the text.
The product of the action of 5′-nucleotidase is adenosine, and I therefore examined whether the latter could be further metabolized by the secreted products of T. spiralis larvae. Secreted proteins were incubated in the presence of adenosine, and spectra were recorded as described in Materials and Methods. A time-dependent shift of the absorbance maximum from 258 to 249 nm was observed (Fig. 5A), as was a decrease at 265 nm accompanied by an increase at 241 nm, shown by the difference spectrum in Fig. 5B, characteristic of the conversion of adenosine to inosine and diagnostic of adenosine deaminase activity (2). The decrease in absorbance at 265 nm, which was linear for 30 min (data not shown), was used to calculate the specific activity of the enzyme at 51.6 ± 3.2 (n = 5) mU/mg of total secreted parasite proteins. The presence of reductant (in the form of DTT) did not affect adenosine deaminase activity, and divalent cations were not essential. Although addition of magnesium ions up to a concentration of 10 mM had no significant effect, 5 mM calcium or zinc was inhibitory. Optimal activities were observed over a broad pH range between the values of pH 6 to 9 (data not shown).
FIG. 5.
Adenosine deaminase activity in T. spiralis secreted products. (A) UV spectra were recorded over a period of 1 h following the addition of 100 μM adenosine into the reaction medium. (B) Difference spectra of the data shown in panel A obtained by subtraction of each spectrum from that at time zero.
Secreted products of adult parasites recovered from the intestines of rats 4 days postinfection were also examined for these enzymes, and levels of activities comparable to those found in larval products were obtained (data not shown).
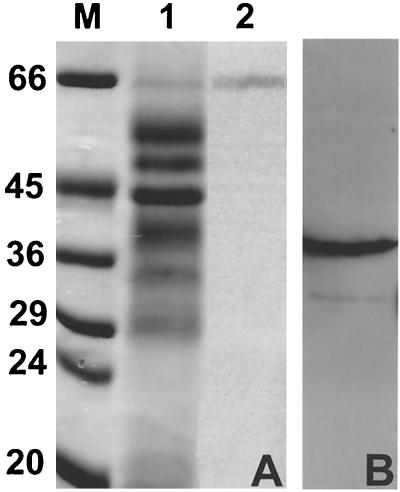
An attempt was made to correlate the observed enzymatic activities with specific proteins present in larval secreted products. We had previously adopted an affinity purification protocol in order to isolate a secreted nucleoside diphosphate kinase and noted another protein present in the fraction which was eluted from the column with 2 mM ATP (20). The procedure was repeated, and a single protein with an apparent molecular mass of 67 kDa was eluted with ATP (Fig. 6A). This fraction contained approximately 0.006% of the total secreted products, which therefore precluded biochemical characterization. The protein was subjected to N-terminal sequencing, however, which yielded the amino acid sequence XQLTLIHTND. A BLAST search on this sequence identified strong similarities with 5′-nucleotidases from a diverse range of species.
FIG. 6.
Identification of the 5′-nucleotidase and adenosine deaminase in T. spiralis secreted products. (A) SDS-PAGE following purification of the 5′-nucleotidase by ATP affinity chromatography. Lane 1, total secreted products; lane 2, protein fraction eluted with 2 mM ATP. (B) Western blot of secreted products reacted with an antibody to mammalian adenosine deaminase. The molecular masses of marker proteins (M) are shown in kilodaltons.
Total secreted proteins were probed with an antibody raised against mammalian adenosine deaminase. Strong reactivity occurred to a single protein with an apparent molecular mass of 38 kDa (Fig. 6B), and it is therefore assumed that this represents the secreted adenosine deaminase which was characterized biochemically (Fig. 5).
DISCUSSION
It is shown that T. spiralis infective larvae secrete proteins which constitute an enzymatic cascade for the metabolism of extracellular nucleotides. To the best of my knowledge, secretion of these enzymes from a parasitic nematode has not been previously reported. Secreted products convert nucleoside 5′-diphosphates to the corresponding monophosphates via the action of an apyrase. This enzyme catalyzed the hydrolysis of ADP and UDP, and under our conditions, UDP was the preferred substrate. We have been unable as yet to correlate the activity with a particular protein. Significant hydrolysis of ATP or any other nucleoside triphosphate was not observed. This is unusual, as the majority of the members of the apyrase enzyme family catalyze the hydrolysis of both nucleoside 5′-triphosphates and nucleoside 5′-diphosphates, although with different preferences for the type of nucleotide (64). The enzyme activity present in T. spiralis secreted products appears to be more similar to a protein belonging to a nucleoside 5′-diphosphate specific subclass, referred to as NTPDase5, which has recently been characterized and denoted CD39-L4 (45, 46). CD39-L4 is a human apyrase which, although it contains the five apyrase conserved regions shared by all members of the family (52), lacks the C-terminal hydrophobic domain. The enzyme is secreted, and although it efficiently hydrolyses ADP, it shows a strong preference for UDP as does the apyrase in T. spiralis secreted products reported here. CD39-L4 is expressed in macrophages (45, 46), and several suggestions have been put forward regarding its possible function. It was postulated that macrophages at sites of vascular injury secrete this enzyme, degrading ADP and thus attenuating platelet aggregation. Alternatively, it has been suggested (45) that the preference for UDP could be linked to modification of the levels of extracellular UDP, which specifically activates the P2Y6 receptor (9). The latter receptor is expressed on all leukocytes and in particular on infiltrating T cells present during some inflammatory states (54), although the biological consequence of receptor engagement in T cells is not understood. Interestingly, it has been shown that monocytes are stimulated by extracellular UDP acting via the P2Y6 receptor, to produce interleukin 8 (IL-8), a chemoattractant for neutrophils. It was further demonstrated that IL-8 production was inhibited when extracellular UDP was degraded by a heterologous apyrase. (59). The enzyme present in the secreted products of T. spiralis larvae hydrolyses ADP and UDP and could therefore prevent both ADP and UDP receptor activation. Thus, this secreted apyrase could inactivate, via degradation of ADP, P2Y1/P2Y12 receptors involved in platelet aggregation or could, via degradation of UDP, inactivate the P2Y6 receptor functions mediated by this nucleoside on a variety of leukocytes.
T. spiralis larvae also secrete a 5′-nucleotidase enzyme which converts AMP to adenosine. This enzyme was identified as a protein with an apparent molecular mass of 67 kDa and the N-terminal amino acid sequence XQLTLIHTND. A BLAST search using this sequence indicated high levels of similarity with mammalian and insect 5′-nucleotidases. Enzymes in the 5′-nucleotidase family are characterized by five to seven conserved sequence regions (58), and indeed the N-terminal sequence of the T. spiralis protein contains one of these regions, which is considered a 5′-nucleotidase signature (LTLIHTND). A search of the T. spiralis EST database using the N-terminal protein sequence indicated identity with an ORF from two overlapping cDNAs (GenBank accession numbers BG353625 and BG302003). This ORF shows homology to 5′-nucleotidases from diverse sources, although the ESTs are partial sequences corresponding to less than 20% of a predicted full-length protein. Nevertheless, the ORF predicted a 21-amino-acid hydrophobic signal peptide immediately preceding the N-terminal sequence of the mature protein.
Vertebrate 5′-nucleotidase enzymes have been subdivided into various classes depending on their cellular origin, substrate specificities, pH optima, and cation requirements (63). Membrane-bound forms of 5′-nucleotidases are known to be surface-located ecto-enzymes linked to the lipid bilayer via a glycosyl phosphatidylinositol (GPI) anchor. The soluble enzymes fall into three classes: two are truly cytosolic, with Km values in the millimolar range, and are subdivided into AMPases and IMPases depending on the substrate preference. Another soluble form has also been identified which results from cleavage of the GPI-linked ecto-enzyme by phosphatidylinositol-specific phospholipase C (63, 64) and has properties identical to those of the membrane-anchored enzyme. A different type of soluble 5′-nucleotidase with properties distinct from those of the above-mentioned classes has been found in human seminal plasma (44), and a 5′-nucleotidase, additionally exhibiting phosphodiesterase activity, has been reported to occur in the salivary glands of the hematophagous sandfly Lutzomyia longipalis (7, 50). The kinetic properties, substrate specificity, and sensitivity to various compounds of the T. spiralis 5′-nucleotidase indicate that this enzyme is distinct from previously reported members of this enzyme family. Concanavalin A and p[CH2]pA are specific and potent inhibitors of ecto-5′-nucleotidases (65) and the soluble forms resulting from cleavage of the GPI anchor and have been used to distinguish these forms from the cytosolic variants. Interestingly, although the enzyme was inhibited by p[CH2]pA, it was relatively resistant to concanavalin A, again suggesting that it is not a typical member of its class. Although the possibility that the T. spiralis enzyme is recovered in secreted products following phospholipase cleavage of a membrane-bound form cannot be categorically excluded, this is unlikely not only due to its properties but also because it is secreted on a daily basis at a constant rate over the term of culture.
The product of the enzymatic action of 5′-nucleotidases is adenosine, which can potentially be further metabolized by the enzyme adenosine deaminase. The presence of this protein in the T. spiralis-secreted products was demonstrated by biochemical analysis and confirmed by Western blotting with an antiserum to a bovine enzyme. Adenosine deaminases catalyze the irreversible hydrolytic deamination of adenosine to inosine and ammonia. This enzyme class has a broad phylogenetic distribution, with amino acid sequences being highly conserved from bacteria to mammals (11). The properties of the T. spiralis adenosine deaminase appear to be similar to those from heterologous sources. Adenosine deaminase is typically a cytosolic enzyme present in all mammalian tissues, although in recent years it has also been found in the salivary glands of hematophagous arthropods (6, 38, 51). As activity was found to be reduced following a blood meal, it has been suggested that the enzyme in these insects is secreted.
Recently extracellular adenosine deaminases have also been shown to exist on the surfaces of hematopoietic cells, and a surface-bound variant on lymphocytes is known to bind to the T-cell activation marker CD26 (19). It has been proposed that this protects T cells from an adenosine-mediated inhibition of proliferation and regulates the ability of CD26 to promote cytokine production.
Adenosine and inosine, the products of the 5′-nucleotidase and adenosine deaminase, respectively, are well-known regulators of inflammation and have been shown to have immunomodulatory effects (12, 39). Adenosine not only inhibits platelet aggregation but suppresses the production of inflammatory cytokines, such as IL-1, IL-12, tumor necrosis factor alpha, and macrophage inflammatory protein 1α, by monocytes and macrophages and enhances IL-10 production (25, 30, 31, 40). Inosine has also been shown to suppress inflammatory cytokine synthesis (26, 41). The role of ADP in platelet aggregation is well characterized, whereas that of extracellular ATP is more complex and can have both proinflammatory and anti-inflammatory effects. Thus, although ATP can trigger mast cell chemotaxis and the oxidative burst in neutrophils (33, 42), it is also known to suppress IL-12 and TNF-α release from macrophages but to enhance release of IL-10 (24).
Mucosal mast cells have been demonstrated to be important effector cells against several intestinal nematodes, notably T. spiralis (22, 23). The mechanisms whereby mast cells contribute to expulsion are unclear but may include local production of cytokines and other inflammatory mediators or secretion of mucosal mast cell-specific serine proteases. Deletion of the mouse mast cell protease-1 gene is associated with significantly delayed expulsion of T. spiralis, demonstrating a role for a defined mast cell product in immunity to enteric nematodes (32). Interestingly, it is unclear how mast cells are activated during nematode infection, as degranulation occurs in the absence of the classical IgE/FcεR1 interaction (21), but this has also been reported to be modulated by P1 receptor activation (39, 55).
Most blood-sucking arthropods contain large amounts of nucleotide-metabolizing enzymes in their saliva, such as ATPase, apyrase, 5′-nucleotidase, and adenosine deaminase (5, 6, 7, 50, 51, 56, 57), as nucleotides are released in high concentrations from injured cells during feeding, and the repertoire of enzymes is predicted to act so as to modulate host hemostasis, inflammation, and immunity (49). Bacterial pathogens such as Mycobacterium bovis, Pseudomonas aeruginosa, Burkholderia cepacia, and Vibrio cholerae also secrete a range of nucleotide-metabolizing enzymes, including ATPase, 5′-nucleotidase, adenylate kinase, and NDPK (43, 60, 61, 62). It has been suggested that these enzymes interfere with purinergic signaling on mast cells and macrophages through alterations in the availability of agonist molecules.
The absence of an ATPase activity in T. spiralis secreted products was initially surprising, as this would indicate that extracellular ATP is not degraded by proteins in the secreted products. Although this may well be the case, it is worth noting, as we have previously shown, that T. spiralis larvae secrete serine/threonine kinases and a nucleoside diphosphate kinase (3, 20). These enzymes would utilize ATP, and importantly, NDPK would convert ATP to ADP or UTP to UDP in the presence of suitable nucleoside phosphate acceptors.
At this stage we can only speculate on the role of these enzymes secreted by T. spiralis. As they are secreted rather than membrane-bound enzymes it is unlikely that they have a function in nucleoside salvaging. Nevertheless, they could modulate both the availability and local concentrations of nucleotides and would therefore be expected to control purine/pyrimidine receptor activation with concomitant regulatory effects on inflammatory responses occurring during host cell invasion. A model diagram of the cascade catalyzed by the secreted proteins showing the potential fate of extracellular nucleotides and some of the cells likely to be affected is shown in Fig. 7.
FIG. 7.
A simplified model diagram of the enzymatic cascade catalyzed by the secreted proteins of T. spiralis. Extracellular nucleotides can be either available for receptor binding or metabolized by the enzymes identified. The type, local concentration, and availability of nucleotides may thus be modulated by the parasite. For simplicity, only receptors on hematopoietic cells without subtype-specific distribution are indicated, and cell membrane-bound ecto-enzymes involved in nucleotide degradation are not included. Details are described in the text.
The fact that bacterial pathogens and hematophagous insects, the latter inducing extensive host tissue damage, also secrete similar enzymes suggests that this may be a conserved but as yet underappreciated feature of many organisms with important consequences for pathogenicity. Different types of purinergic receptors may regulate different facets of immune and inflammatory responses, and it is possible that the parasite can modulate these to its own advantage. We are currently investigating these processes.
Acknowledgments
This work was supported by the Wellcome Trust through a Research Leave Award.
I am indebted to Murray Selkirk for his continuous support and encouragement.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abbracchio, M. P., and G. Burnstock. 1994. Purinoreceptors: are there families of P2X and P2Y purinoreceptors? Pharmacol. Ther. 64:445-475. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal, P. R., and R. E. Parks. 1978. Adenosine deaminase from human erythrocytes. Methods Enzymol. 51:502-507. [DOI] [PubMed] [Google Scholar]
- 3.Arden, S. R., A. M. Smith, M. J. Booth, S. Tweedie, K. Gounaris, and M. E. Selkirk. 1997. Identification of serine/threonine protein kinases secreted by Trichinella spiralis infective larvae. Mol. Biochem. Parasitol. 90:111-119. [DOI] [PubMed] [Google Scholar]
- 4.Baykov, A. A., O. A. Evtushenko, and S. M. Avaeva. 1988. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal. Biochem. 171:266-270. [DOI] [PubMed] [Google Scholar]
- 5.Champagne, D. E., C. T. Smart, J. M. C. Ribeiro, and A. A. James. 1995. The salivary gland-specific apyrase of the mosquito Aedes aegypti is a member of the 5′-nucleotidase family. Proc. Natl. Acad. Sci. USA 92:694-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlab, R., E. D. Rowton, and J. M. C. Ribeiro. 2000. The salivary adenosine deaminase from the sand fly Lutzomyia longipalpis. Exp. Parasitol. 95:45-53. [DOI] [PubMed] [Google Scholar]
- 7.Charlab, R., J. G. Valenzuela, E. D. Rowton, and J. M. C. Ribeiro. 1999. Toward an understanding of the biochemical and pharmacological complexity of the saliva of a hematophagous sand fly Lutzomyia longipalpis. Proc. Natl. Acad. Sci. USA 96:15155-15160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christoforidis, S., T. Papamarcaki, D. Galaris, R. Kellner, and O. Tsolas. 1995. Purification and properties of human placental ATP diphosphohydrolase. Eur. J. Biochem. 234:66-74. [DOI] [PubMed] [Google Scholar]
- 9.Communi, D., M. Parmentier, and J. M. Boeynaems. 1996. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem. Biophys. Res. Commun. 222:303-308. [DOI] [PubMed] [Google Scholar]
- 10.Communi, D., N. S. Gonzalez, M. Detheux, S. Brezillon, V. Lannoy, M. Parmentier, and J.-M. Boeynaems. 2001. Identification of a novel human ADP receptor coupled to Gi. J. Biol. Chem. 276:41479-41485. [DOI] [PubMed] [Google Scholar]
- 11.Cristalli, G., S. Costanzi, C. Lambertucci, G. Lupidi, S. Vittori, R. Volpini, and E. Camaioni. 2001. Adenosine deaminase: functional implications and different classes of inhibitors. Med. Res. Rev. 21:105-128. [DOI] [PubMed] [Google Scholar]
- 12.Cronstein, B. N. 1994. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 76:5-13. [DOI] [PubMed] [Google Scholar]
- 13.Despommier, D. D. 1983. Biology, p. 75-151. In W. C. Campbell (ed.), Trichinella and Trichinosis. Plenum Press, New York, N.Y.
- 14.Di Virgilio, F., D. Ferrari, S. Falzoni, P. Chiozzi, M. Munerati, T. H. Steinberg, and O. R. Baricordi. 1996. P2 purinoceptors in the immune system. Ciba Found. Symp. 198:290-302. [DOI] [PubMed] [Google Scholar]
- 15.Di Virgilio, F., P. A. Borea, and P. Illes. 2001. P2 receptors meet the immune system. Trends Pharmacol. Sci. 22:5-7. [DOI] [PubMed] [Google Scholar]
- 16.Di Virgilio, F., P. Chiozzi, D. Ferrari, S. Falzoni, J. M. Sanz, A. Morelli, M. Torboli, G. Bolognesi, and O. R. Baricordi. 2001. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97:587-600. [DOI] [PubMed] [Google Scholar]
- 17.Di Virgilio, F., S. Falconi, C. Mutini, J. M. Sanz, and P. Chiozzi. 1998. Purinergic P2X7 receptor: a pivotal role in inflammation and immunomodulation. Drug Dev. Res. 45:207-213. [Google Scholar]
- 18.Finkelman, F. D., and J. F. Urban, Jr. 2001. The other side of the coin: the protective role of the Th2 cytokines. J. Allergy Clin. Immunol. 107:772-780. [DOI] [PubMed] [Google Scholar]
- 19.Gorrell, M. D., V. Gysbers, and G. W. McCaughan. 2001. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand. J. Immunol. 54:249-264. [DOI] [PubMed] [Google Scholar]
- 20.Gounaris, K., S. Thomas, P. Najarro, and M. E. Selkirk. 2001. Secreted variant of nucleoside diphosphate kinase from the intracellular parasitic nematode Trichinella spiralis. Infect. Immun. 69:3658-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grencis, R. K. 1997. Th2-mediated host protective immunity to intestinal nematode infections. Philos. Trans. R. Soc. Lond. B Biol. Sci. 352:1377-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grencis, R. K., K. J. Else, J. F. Huntley, and S. I. Nishikawa. 1993. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol. 15:55-59. [DOI] [PubMed] [Google Scholar]
- 23.Ha, T. Y., N. D. Reed, and P. K. Crowle. 1983. Delayed expulsion of adult Trichinella spiralis by mast cell deficient W/Wv mice. Infect. Immun. 41:445-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasko, G., D. G. Kuhel, A. L. Salzman, and C. Szabo. 2000. ATP suppression of interleukin-12 and tumour necrosis factor-α release from macrophages. Br. J. Pharmacol. 129:909-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasko, G., D. G. Kuhel, J. F. Chen, M. A. Schwarzschild, E. A. Deitch, J. G. Mabley, A. Marton, and C. Szabo. 2000. Adenosine inhibits IL-12 and TNF-α production via adenosine A2A receptor-dependent and independent mechanisms. FASEB J. 14:2065-2074. [DOI] [PubMed] [Google Scholar]
- 26.Hasko, G., D. G. Kuhel, Z. H. Nemeth, J. G. Mabley, R. F. Stachlewitz, L. Virag, Z. Lohinai, G. J. Southan, A. L. Salzman, and C. Szabo. 2000. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J. Immunol. 164:1013-1019. [DOI] [PubMed] [Google Scholar]
- 27.Hollopeter, G., H. M. Jantzen, D. Vincent, G. Li, L. England, V. Ramakrishnan, R. B. Yang, P. Nurden, A. Nurden, D. Julius, and P. B. Conley. 2001. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409:202-207. [DOI] [PubMed] [Google Scholar]
- 28.Itaya, K., and M. Ui. 1966. A new micromethod for the colorimetric determination of inorganic phosphate. Clin. Chim. Acta 14:361-366. [DOI] [PubMed] [Google Scholar]
- 29.Jin, J., V. R. Dasari, F. D. Sistare, and S. P. Kunapuli. 1998. Distribution of P2Y receptor subtypes on haematopoietic cells. Br. J. Pharmacol. 123:789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawashima, Y., T. Nagasawa, and H. Ninomiya. 2000. Contribution of ecto-5′-nucleotidase to the inhibition of platelet aggregation by human endothelial cells. Blood 96:2157-2162. [PubMed] [Google Scholar]
- 31.Khoa, N. D., M. C. Montesinos, A. B. Reiss, D. Delano, N. Awadallah, and B. N. Cronstein. 2001. Inflammatory cytokines regulate function and expression of adenosine A2A receptors in human monocytic THP-1 cells. J. Immunol. 167:4026-4032. [DOI] [PubMed] [Google Scholar]
- 32.Knight, P. A., S. H. Wright, C. E. Lawrence, Y. Y. Paterson, and H. R. Miller. 2000. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 192:1849-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuroki, M., and S. Minakami. 1989. Extracellular ATP triggers superoxide production in human neutrophils. Biochem. Biophys. Res. Commun. 162:377-380. [DOI] [PubMed] [Google Scholar]
- 34.Lazarowski, E. R., and R. C. Boucher. 2001. UTP as an extracellular signaling molecule. News Physiol. Sci. 16:1-5. [DOI] [PubMed] [Google Scholar]
- 35.Lazarowski, E. R., L. Homolya, R. C. Boucher, and T. K. Harden. 1997. Identification of an ecto-nucleoside diphosphokinase and its contribution to interconversion of P2 receptor agonists. J. Biol. Chem. 272:20402-20407. [DOI] [PubMed] [Google Scholar]
- 36.Lazarowski, E. R., L. Homolya, R. C. Boucher, and T. K. Harden. 1997. Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J. Biol. Chem. 272:24348-24354. [DOI] [PubMed] [Google Scholar]
- 37.Lazarowski, E. R., R. C. Boucher, and T. K. Harden. 2000. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J. Biol. Chem. 275:31061-31068. [DOI] [PubMed] [Google Scholar]
- 38.Li, S., and S. Aksoy. 2000. A family of genes with growth factor and adenosine deaminase similarity are preferentially expressed in the salivary glands of Glosina m. morsitans. Gene 252:83-93. [DOI] [PubMed] [Google Scholar]
- 39.Linden, J. 2001. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 41:775-787. [DOI] [PubMed] [Google Scholar]
- 40.Link, A. A., T. Kino, J. A. Worth, J. L. McGuire, M. L. Crane, G. P. Chrousos, R. L. Wilder, and I. J. Elenkov. 2000. Ligand-activation of the adenosine A2A receptors inhibits IL-12 production by human monocytes. J. Immunol. 164:436-442. [DOI] [PubMed] [Google Scholar]
- 41.Marton, A., P. Pacher, K. G. Murthy, Z. H. Nemeth, G. Hasko, and C. Szabo. 2001. Anti-inflammatory effects of inosine in human monocytes, neutrophils and epithelial cells in vitro. Int. J. Mol. Med. 8:617-621. [PubMed] [Google Scholar]
- 42.McCloskey, M. A., Y. Fan, and S. Luther. 1999. Chemotaxis of rat mast cells toward adenine nucleotides. J. Immunol. 163:970-977. [PubMed] [Google Scholar]
- 43.Melnikov, A., O. Zaborina, N. Dhiman, B. S. Prabhakar, A. M. Chakrabarty, and W. Hendrickson. 2000. Clinical and environmental isolates of Burkholderia cepacia exhibit differential cytotoxicity towards macrophages and mast cells. Mol. Microbiol. 36:1481-1493. [DOI] [PubMed] [Google Scholar]
- 44.Minelli, A., M. Moroni, N. Monacelli, and I. Mezzasoma. 1996. The isolation from human seminal plasma of a new form of soluble 5′-nucleotidase. Biochem. Mol. Med. 58:168-175. [DOI] [PubMed] [Google Scholar]
- 45.Mulero, J. J., G. Yeung, S. T. Nelken, and J. E. Ford. 1999. CD39-L4 is a secreted human apyrase, specific for the hydrolysis of nucleoside diphosphates. J. Biol. Chem. 274:20064-20067. [DOI] [PubMed] [Google Scholar]
- 46.Mulero, J. J., G. Yeung, S. T. Nelken, J. M. Bright, D. W. McGowan, and J. E. Ford. 2000. Biochemical characterisation of CD39L4. Biochemistry 39:12924-12928. [DOI] [PubMed] [Google Scholar]
- 47.Ohta, A., and M. Sitkovsky. 2001. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414:916-919. [DOI] [PubMed] [Google Scholar]
- 48.Ralevic, V., and G. Burnstock. 1998. Receptors for purines and pyrimidines. Pharmacol. Rev. 50:413-492. [PubMed] [Google Scholar]
- 49.Ribeiro, J. M. C. 1995. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect. Agents Dis. 4:143-152. [PubMed] [Google Scholar]
- 50.Ribeiro, J. M. C., E. D. Rowton, and R. Charlab. 2000. The salivary 5′-nucleotidase/phosphodiesterase of the hematophagus sand fly, Lutzomyia longipalpis. Insect Biochem. Mol. Biol. 30:279-285. [DOI] [PubMed] [Google Scholar]
- 51.Ribeiro, J. M. C., R. Charlab, and J. G. Valenzuela. 2001. The salivary adenosine deaminase activity of the mosquitoes Culex quinquefasciatus and Aedes aegypti. J. Exp. Biol. 204:2001-2010. [DOI] [PubMed] [Google Scholar]
- 52.Schulte am Esch, J. S. A., J. Sevigny, E. Kaczmarek, J. B. Siegel, M. Imai, K. Koziak, A. R. Beaudoin, and S. C. Robson. 1999. Structural elements and limited proteolysis of CD39 influence ATP diphosphohydrolase activity. Biochemistry 38:2248-2258. [DOI] [PubMed] [Google Scholar]
- 53.Smith, V. P., M. E. Selkirk, and K. Gounaris. 2000. A reversible protein phosphorylation system is present at the surface of infective larvae of the parasitic nematode Trichinella spiralis. FEBS Lett. 483:104-108. [DOI] [PubMed] [Google Scholar]
- 54.Somers, G. R., F. M. Hammet, L. Trute, M. C. Southey, and D. J. Venter. 1998. Expression of the P2Y6 purinergic receptor in human T cells infiltrating inflammatory bowel disease. Lab. Investig. 78:1375-1383. [PubMed] [Google Scholar]
- 55.Tilley, S. L., V. A. Wagoner, C. A. Salvatore, M. A. Jacobson, and B. H. Koller. 2000. Adenosine and inosine increase cutaneous vasopermeability by activating A3 receptors on mast cells. J. Clin. Investig. 105:361-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valenzuela, J. G., R. Charlab, M. Y. Galperin, and J. M. C. Ribeiro. 1998. Purification, cloning and expression of an apyrase from the bed bug Cimex lectularius. A new type of nucleotide-binding enzyme. J. Biol. Chem. 273:30583-30590. [DOI] [PubMed] [Google Scholar]
- 57.Valenzuela, J. G., Y. Belkaid, E. Rowton, and J. M. C. Ribeiro. 2001. The salivary apyrase of the blood-sucking sand fly Phlebotomus papatasi belongs to the novel Cimex family of apyrases. J. Exp. Biol. 204:229-237. [DOI] [PubMed] [Google Scholar]
- 58.Volknandt, W., M. Vogel, J. Pevsner, Y. Misumi, Y. Ikehara, and H. Zimmermann. 1991. 5′-nucleotidase from the electric ray electric lobe. Primary structure and relation to mammalian and prokaryotic enzymes. Eur. J. Biochem. 202:855-861. [DOI] [PubMed] [Google Scholar]
- 59.Warny, M., S. Aboudola, S. C. Robson, J. Sevigny, D. Communi, S. P. Soltoff, and C. P. Kelly. 2001. P2Y6 nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J. Biol. Chem. 276:26051-26056. [DOI] [PubMed] [Google Scholar]
- 60.Zaborina, O., N. Dhiman, M. L. Chen, J. Kostal, I. A. Holder, and A. M. Chakrabarty. 2000. Secreted products of a nonmucoid Pseudomonas aeruginosa strain induce two modes of macrophage killing: external-ATP-dependent, P2Z-receptor-mediated necrosis and ATP-independent, caspase-mediated apoptosis. Microbiology 146:2521-2530. [DOI] [PubMed] [Google Scholar]
- 61.Zaborina, O., N. Misra, J. Kostal, S. Kamath, V. Kapatral, M. El-Azami El-Idrissi, B. S. Prabhakar, and A. M. Chakrabarty. 1999. P2Z-independent and P2Z receptor-mediated macrophage killing by Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect. Immun. 67:5231-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zaborina, O., X. Li, G. Cheng, V. Kapatral, and A. M. Chakrabarty. 1999. Secretion of ATP-utilising enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol. Microbiol. 31:1333-1343. [DOI] [PubMed] [Google Scholar]
- 63.Zimmermann, H. 1992. 5′-nucleotidase: molecular structure and functional aspects. Biochem. J. 285:345-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmermann, H. 1999. Two novel families of ectonucleotidases: molecular structures, catalytic properties and a search for function. Trends Pharmacol. Sci. 20:231-236. [DOI] [PubMed] [Google Scholar]
- 65.Zimmermann, H. 2000. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg's Arch. Pharmacol. 362:299-309. [DOI] [PubMed] [Google Scholar]