Abstract
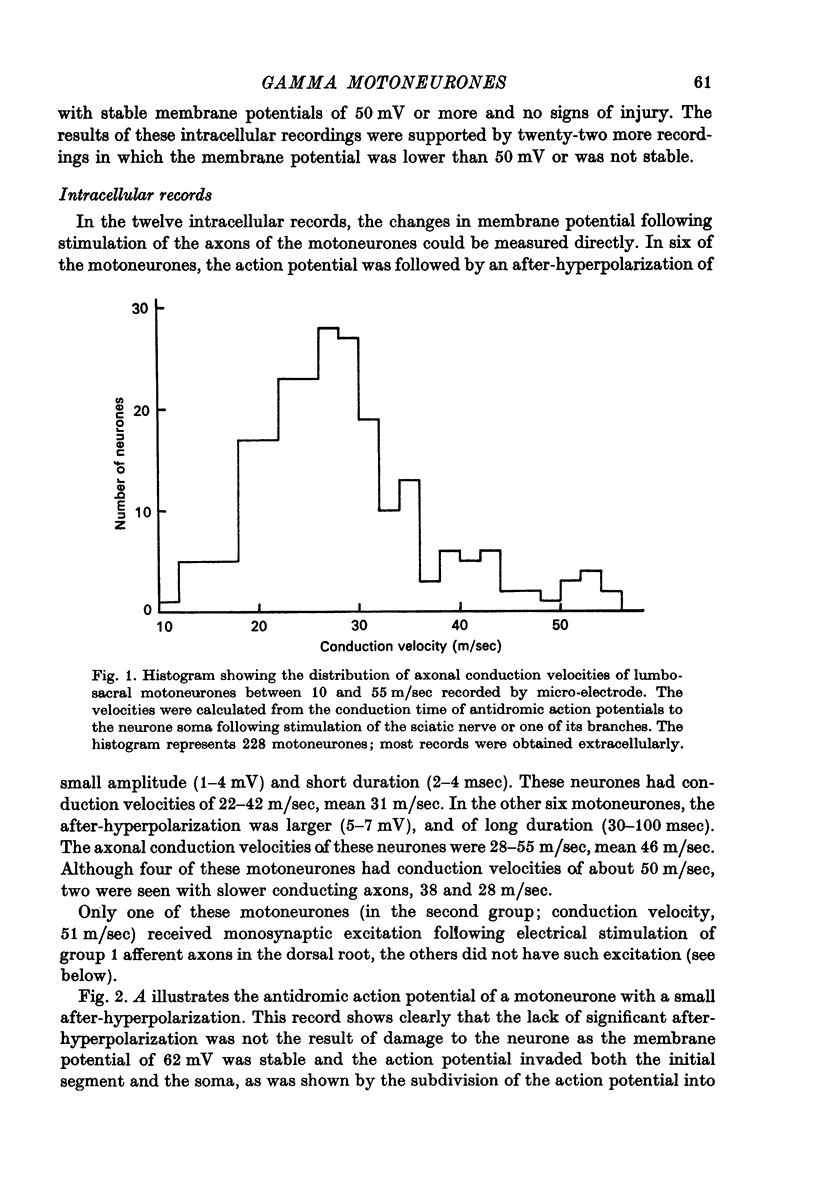
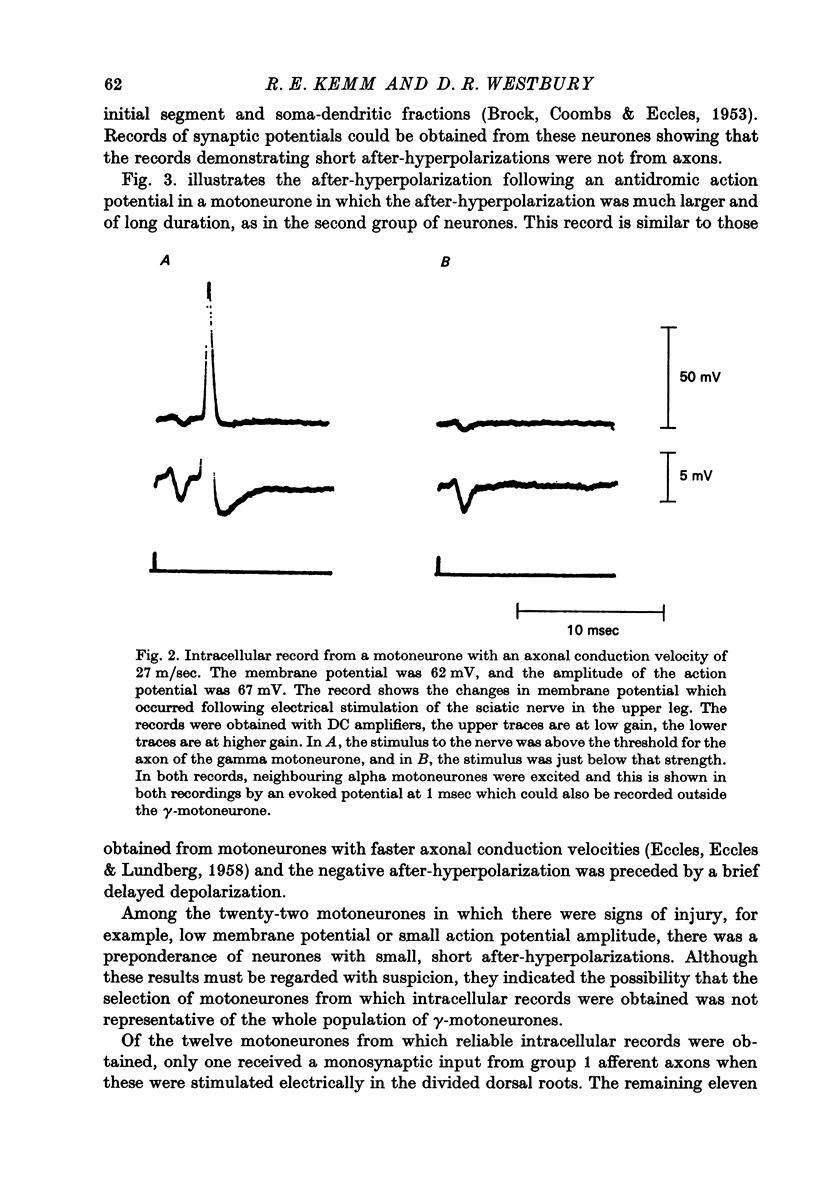
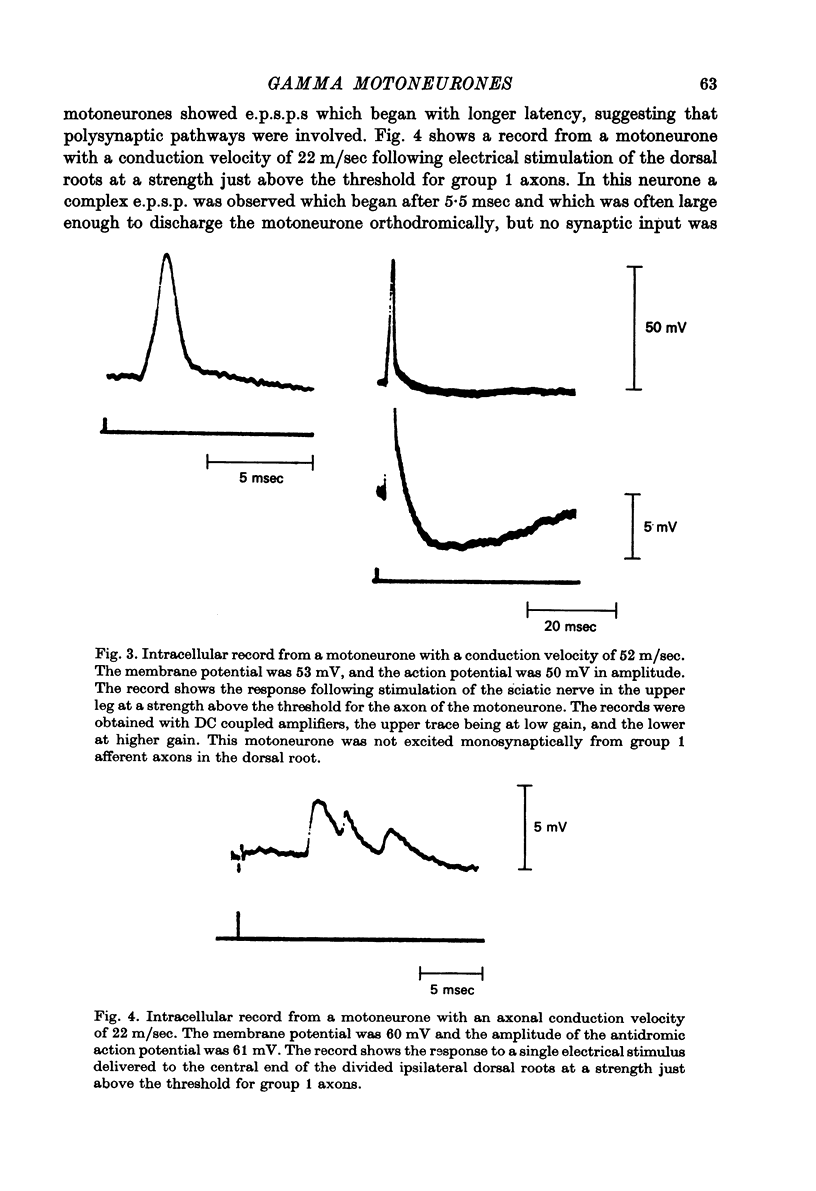
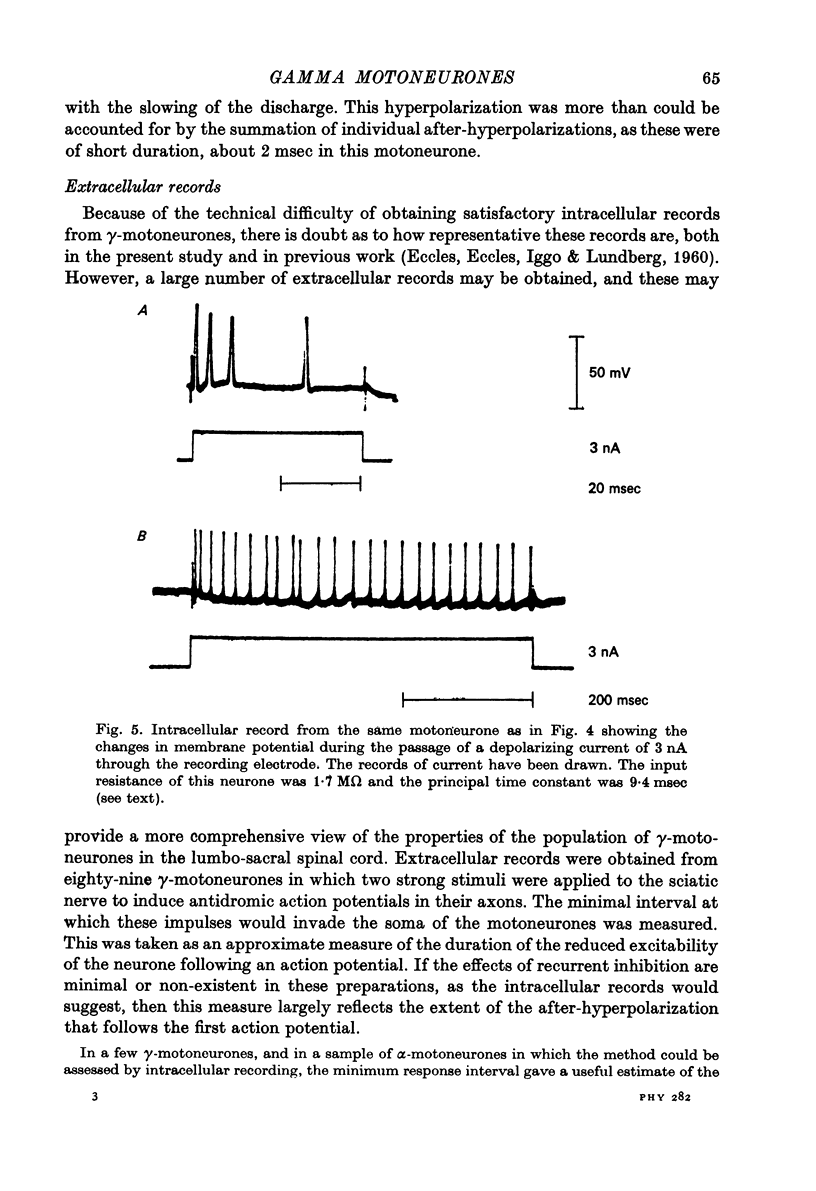
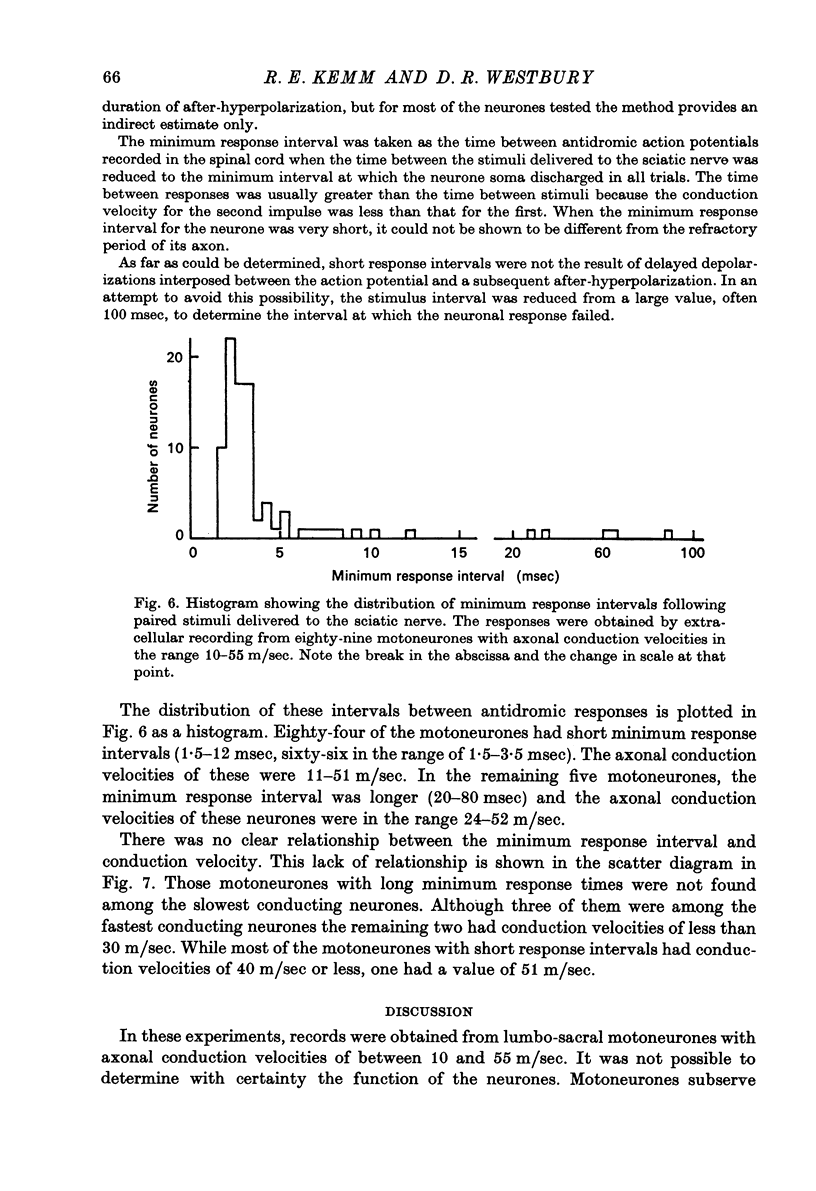
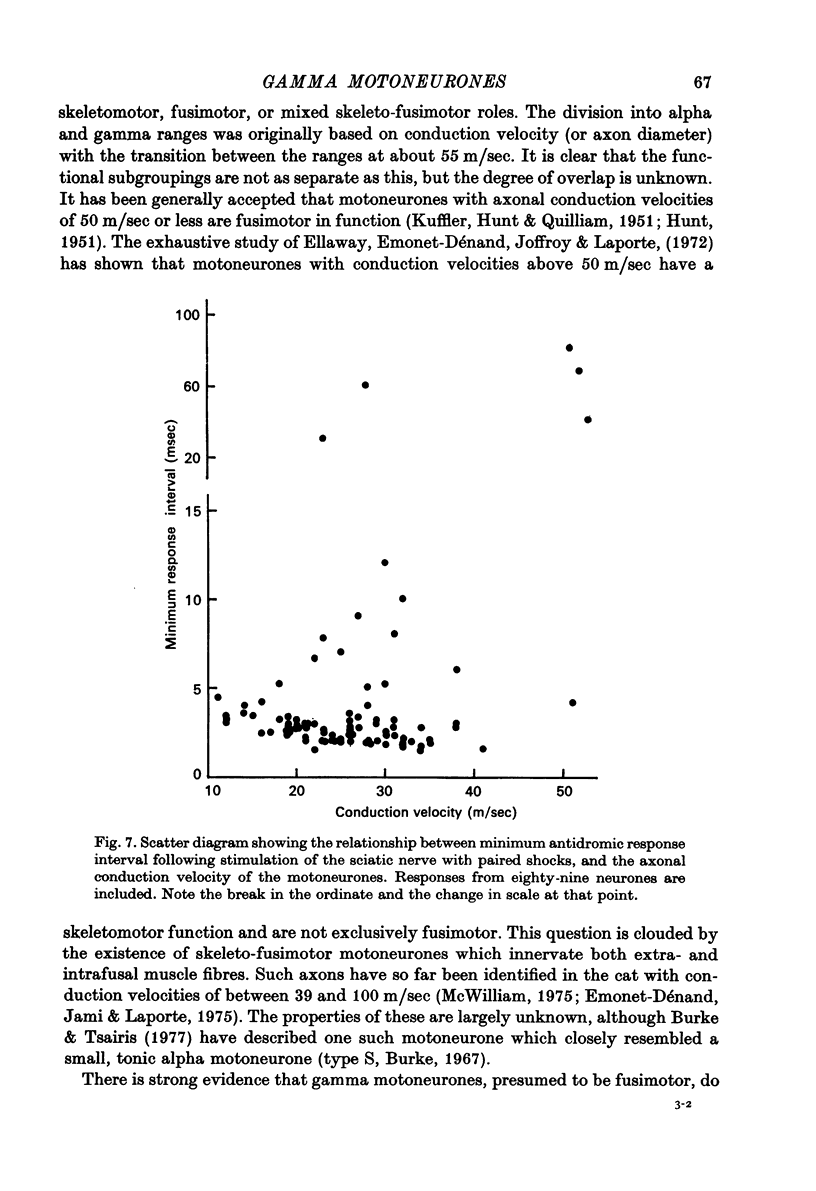
1. Micro-electorde recordings were made from motoneurones in the lumbo-sacral region of the cat spinal cord whose axonal conduction velocities were 10--55 m/sec. Most of these may be presumed to be fusimotor in function. 2. Intracellular records from twelve gamma-motoneurones revealed six with short (2--4 msec) and six with long (30--100 msec) duration after-hyperpolarizations following an antidromically conducted action potential. 3. Using extracellular recording, the excitability of eighty-nine other gamma-motoneurones following an antidromic impulse was tested with a second antidromic action potential. In eighty-four of these neurones, the minimum antidromic response interval was short, 1.5--3.5 msec, implying that in most gamma-motoneurones, after-hyperpolarization was of limited effectiveness and of short duration. In the remaining five neurones, the minimum response interval was longer, 20--80 msec. 4. There was a lack of monosynaptic excitation from group 1 afferent axons in the dorsal roots in eleven of the twelve motoneurones from which intracellular records were obtained. Polysynaptic excitation was commonly observed. 5. In these anaesthetized preparations, there was a lack of recurrent i.p.s.p.s even though such evidence of Renshaw inhibition could be found in the neighbouring alpha-motoneurones. 6. The mean input resistance of gamma-motoneurones was shown to be 1.55 Momega and the principal time constant 8.5 msec by passing hyperpolarizing current through the recording micro-electrode in a bridge circuit. These values are open to error because of the small numbers of neurones investigated, and of the use of the single micro-electrode method. 7. Depolarizing current passed through the recording micro-electrode caused a maintained discharge of action potentials at a high rate. After-hyperpolarization had little effect on discharge rate. The threshold for injected current to cause discharge was very low, and the discharge rate increased rapidly with the magnitude of the current. 8. These properties of gamma-motoneurones arediscussed in relation to their function.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg B., Jeneskog T. Mesencephalic fusimotor control. Exp Brain Res. 1972;15(1):97–112. doi: 10.1007/BF00234960. [DOI] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. Intracellular recording from antidromically activated motoneurones. J Physiol. 1953 Dec 29;122(3):429–461. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F., Gustafsson B. Regulation of repetitive firing in motoneurones by the afterhyperpolarization conductance. Brain Res. 1971 Jul 23;30(2):431–434. doi: 10.1016/0006-8993(71)90096-5. [DOI] [PubMed] [Google Scholar]
- Bergmans J., Grillner S. Reciprocal control of spontaneous activity and reflex effects in static and dynamic flexor gamma-motoneurones revealed by an injection of DOPA. Acta Physiol Scand. 1969 Sep-Oct;77(1):106–124. doi: 10.1111/j.1748-1716.1969.tb04557.x. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Motor unit types of cat triceps surae muscle. J Physiol. 1967 Nov;193(1):141–160. doi: 10.1113/jphysiol.1967.sp008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Histochemical and physiological profile of a skeletofusimotor (beta) unit in cat soleus muscle. Brain Res. 1977 Jul 1;129(2):341–345. doi: 10.1016/0006-8993(77)90013-0. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., LUNDBERG A. Electrophysiological studies on gamma motoneurones. Acta Physiol Scand. 1960 Sep 30;50:32–40. doi: 10.1111/j.1748-1716.1960.tb02070.x. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958 Jul 14;142(2):275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Emonet-Denand F., Joffroy M., Laporte Y. Lack of exclusively fusimotor -axons in flexor and extensor leg muscles of the cat. J Neurophysiol. 1972 Jan;35(1):149–153. doi: 10.1152/jn.1972.35.1.149. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H. Recurrent inhibition of fusimotor neurones exhibiting background discharges in the decerebrate and the spinal cat. J Physiol. 1971 Jul;216(2):419–439. doi: 10.1113/jphysiol.1971.sp009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F., Jami L., Laporte Y. Skeleto-fusimotor axons in the hind-limb muscles of the cat. J Physiol. 1975 Jul;249(1):153–166. doi: 10.1113/jphysiol.1975.sp011008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F., Laporte Y., Matthews P. B., Petit J. On the subdivision of static and dynamic fusimotor actions on the primary ending of the cat muscle spindle. J Physiol. 1977 Jul;268(3):827–861. doi: 10.1113/jphysiol.1977.sp011884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm C., Noth J. Reflex responses of gamma motoneurones to vibration of the muscle they innervate. J Physiol. 1976 Mar;256(1):117–136. doi: 10.1113/jphysiol.1976.sp011315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Supraspinal and segmental control of static and dynamic gamma-motoneurones in the cat. Acta Physiol Scand Suppl. 1969;327:1–34. [PubMed] [Google Scholar]
- HUNT C. C., PAINTAL A. S. Spinal reflex regulation of fusimotor neurones. J Physiol. 1958 Sep 23;143(2):195–212. doi: 10.1113/jphysiol.1958.sp006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. The reflex activity of mammalian small-nerve fibres. J Physiol. 1951 Dec 28;115(4):456–469. doi: 10.1113/jphysiol.1951.sp004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., HUNT C. C., QUILLIAM J. P. Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J Neurophysiol. 1951 Jan;14(1):29–54. doi: 10.1152/jn.1951.14.1.29. [DOI] [PubMed] [Google Scholar]
- Kemm R. E., Westbury D. R. The after-hyperpolarization of gamma motoneurones [proceedings]. J Physiol. 1976 Dec;263(1):124P–125P. [PubMed] [Google Scholar]
- RALL W. Membrane potential transients and membrane time constant of motoneurons. Exp Neurol. 1960 Oct;2:503–532. doi: 10.1016/0014-4886(60)90029-7. [DOI] [PubMed] [Google Scholar]
- Sjöström A., Zangger P. Muscle spindle control during locomotor movements generated by the deafferented spinal cord. Acta Physiol Scand. 1976 Jul;97(3):281–291. doi: 10.1111/j.1748-1716.1976.tb10265.x. [DOI] [PubMed] [Google Scholar]
- Trott J. R. The effect of low amplitude muscle vibration on the discharge of fusimotor neurones in the decerebrate cat. J Physiol. 1976 Mar;255(3):635–649. doi: 10.1113/jphysiol.1976.sp011300. [DOI] [PMC free article] [PubMed] [Google Scholar]


