Abstract
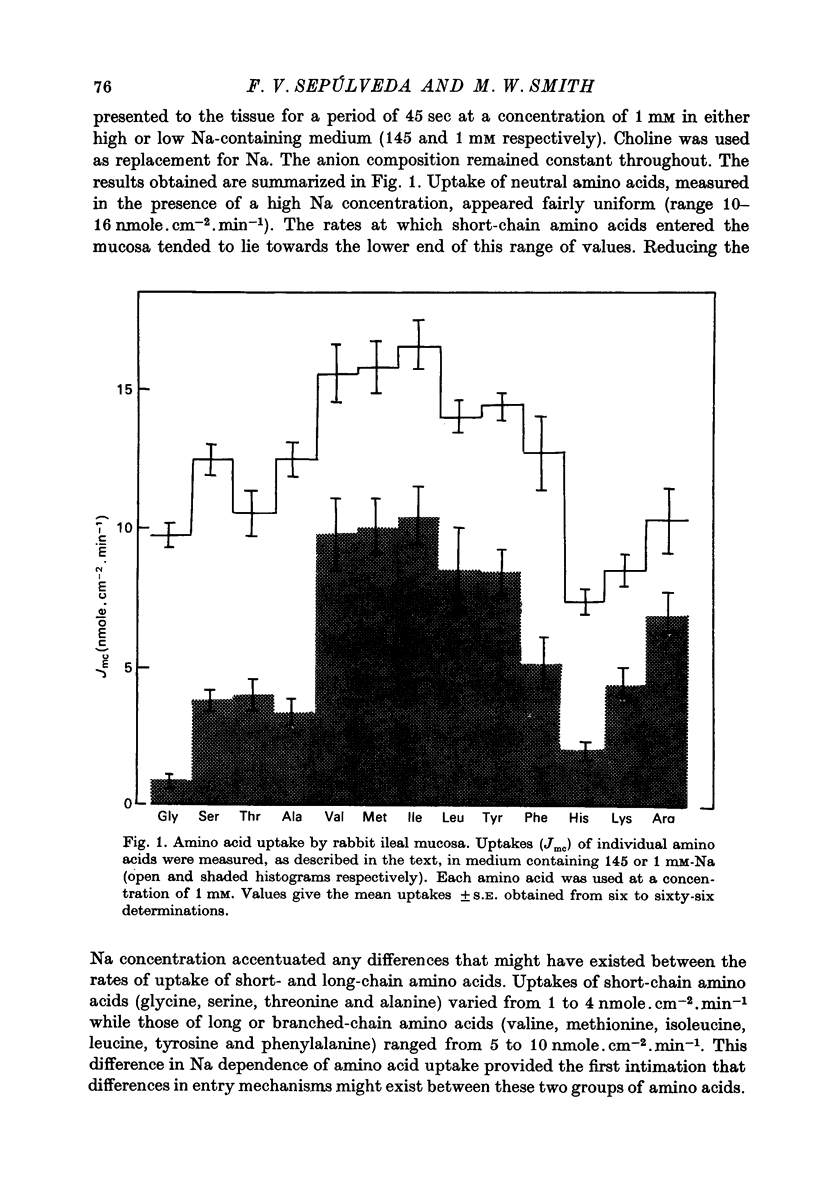
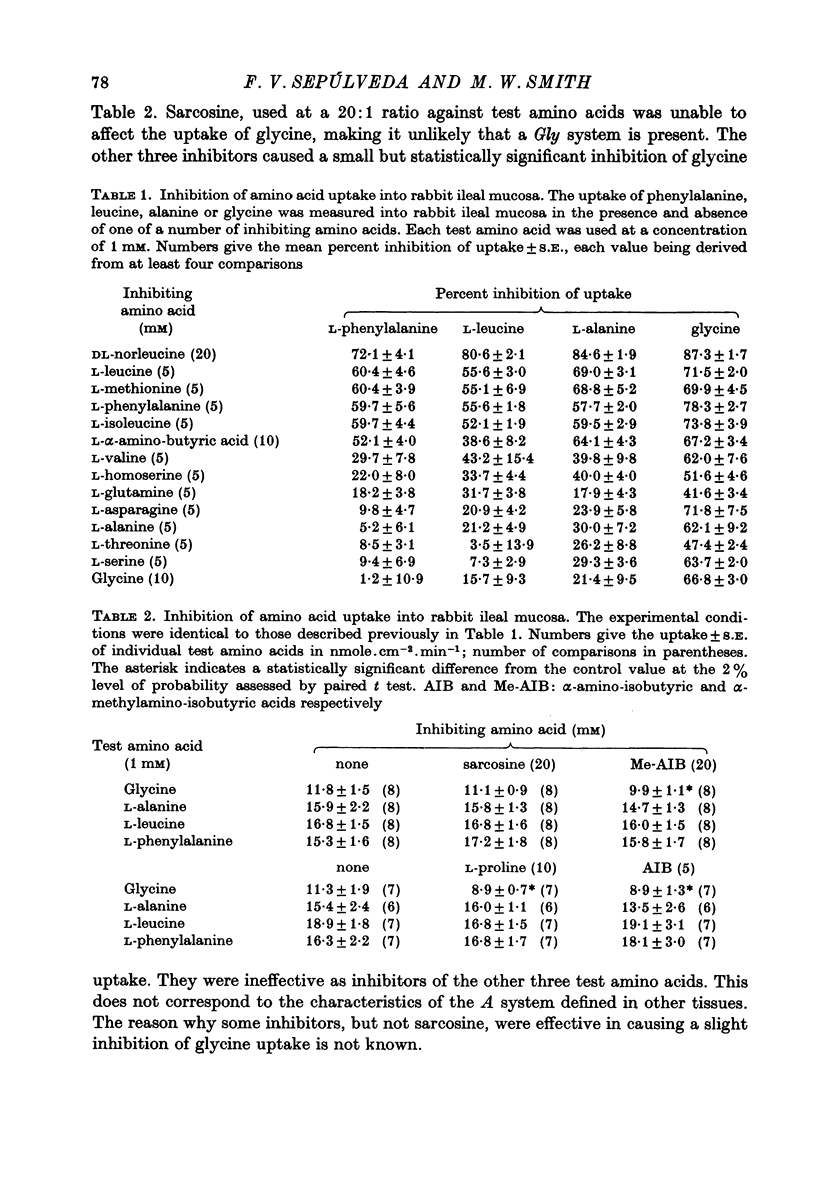
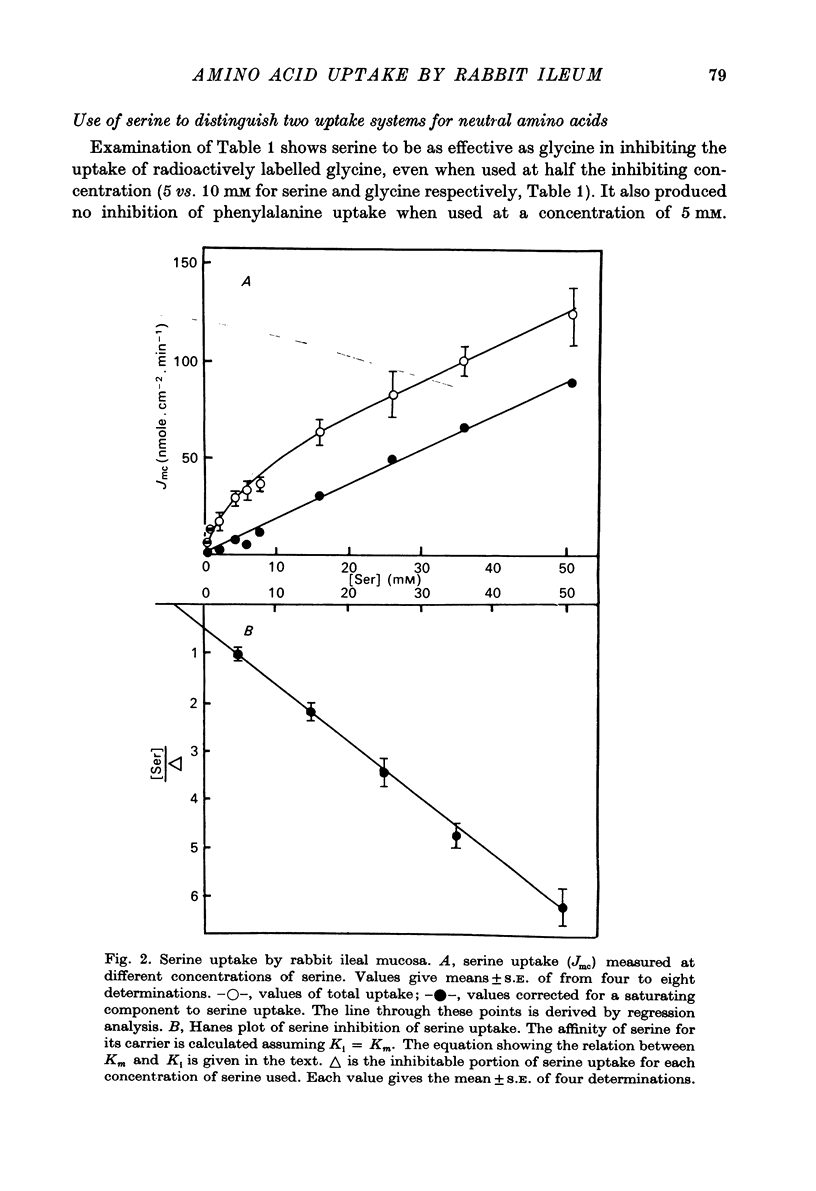
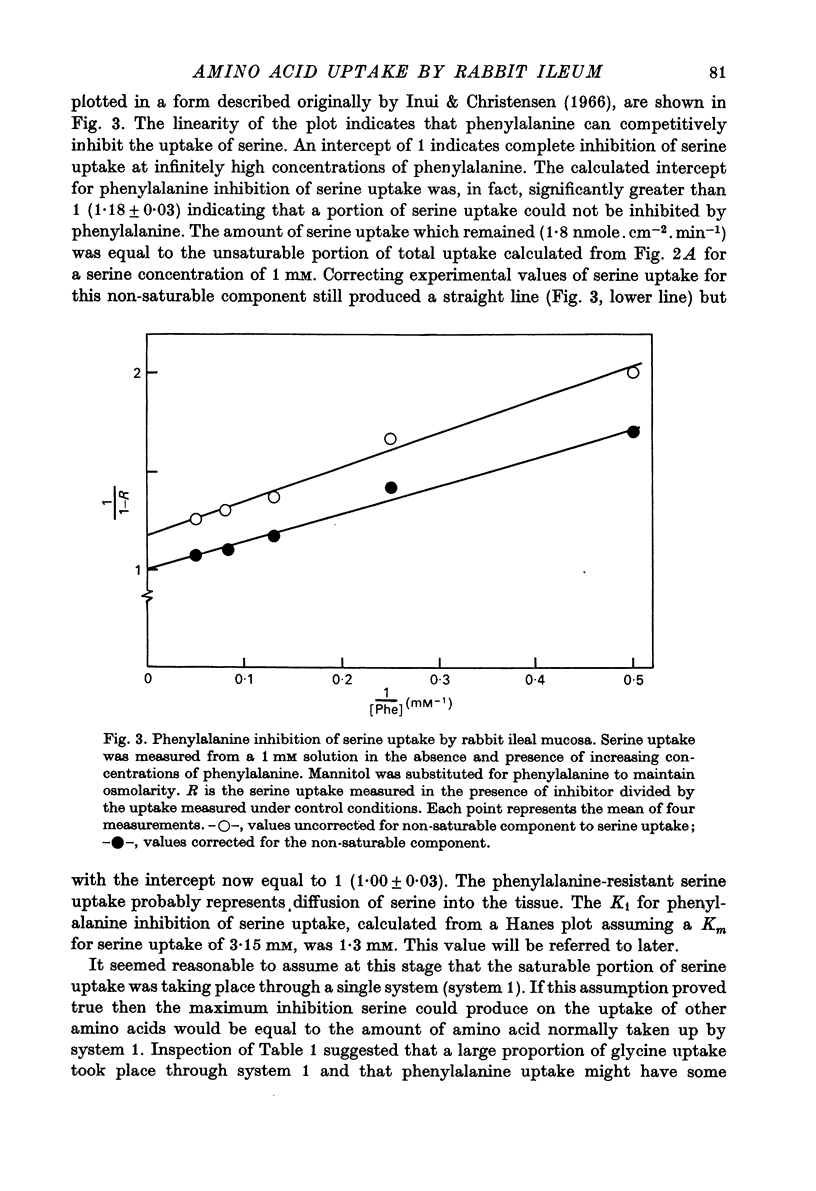
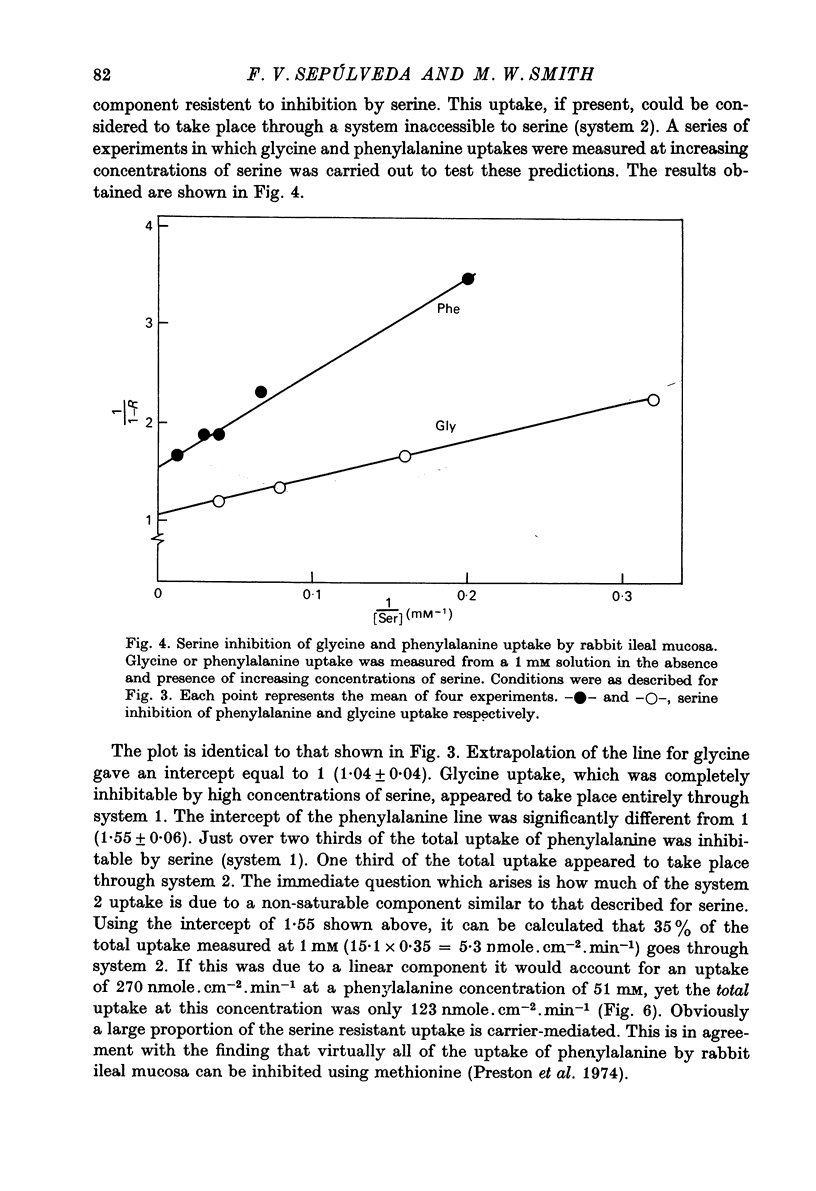
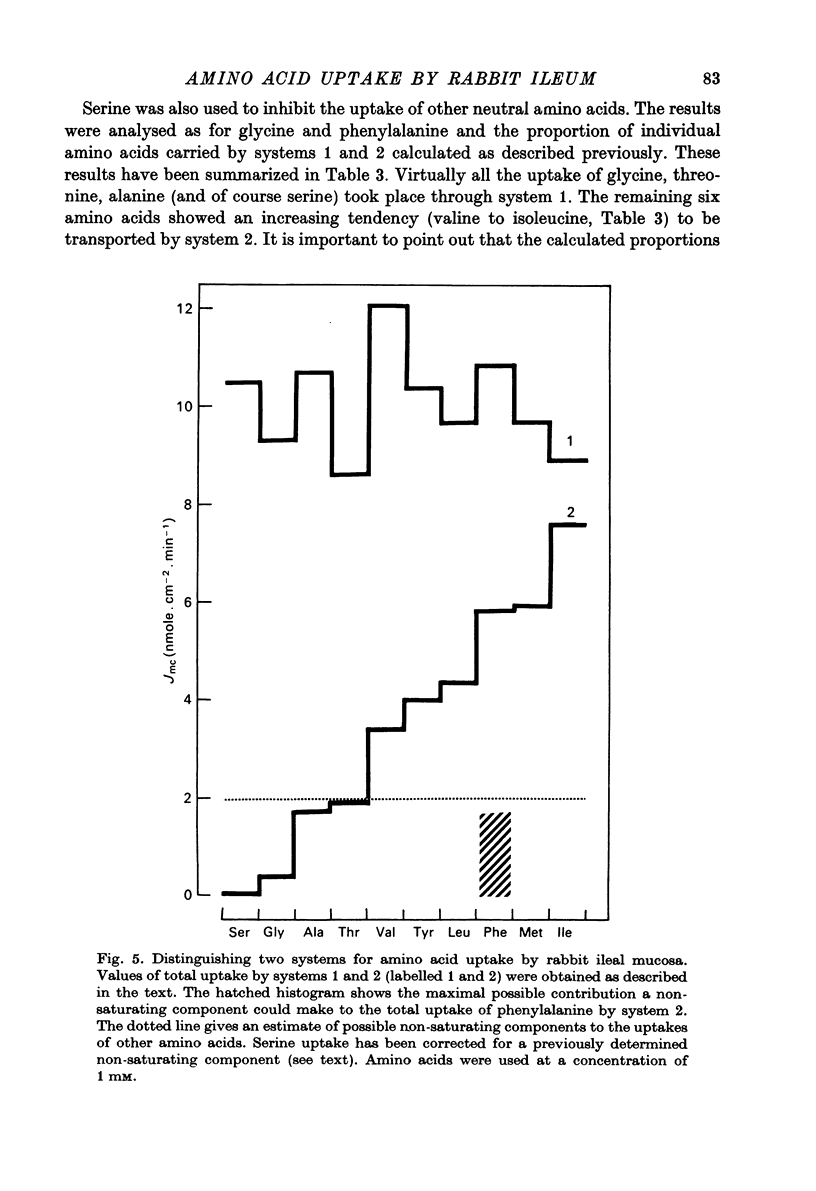
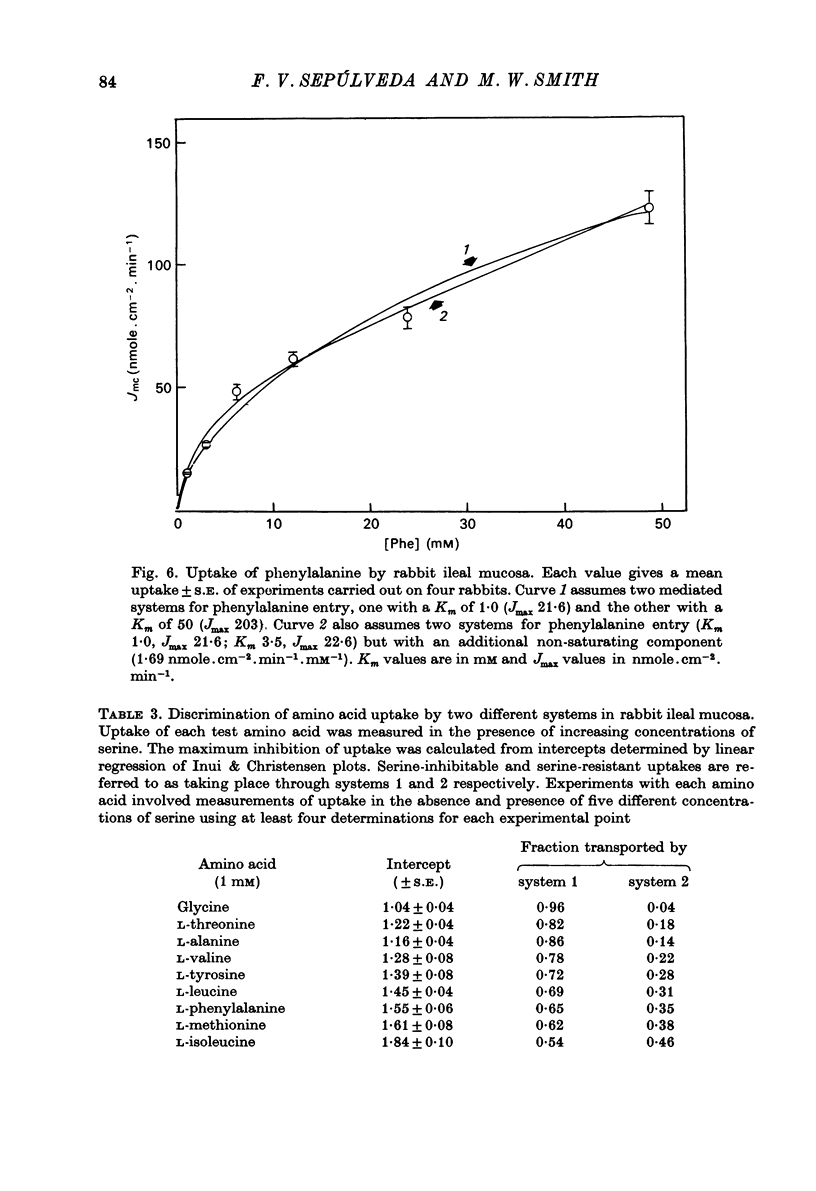
1. Influx of neutral and basic amino acids into the mucosal epithelium of the rabbit ileum was measured in the presence of high and low concentrations of Na. Uptake measured at an amino acid concentration of 1 mM varied from 10 to 16 nmole . cm-2 . min-1. Removal of Na inhibited the uptake of short-chain amino acids more than long-chain amino acids. 2. Inhibition of uptake of glycine, alanine, leucine and phenylalanine by a selection of non-radioactive amino acids was found to follow a particular pattern. Long-chain amino acids inhibited the uptake of all test amino acids; short-chain amino acids inhibited preferentially the uptake of glycine. 3. The maximum inhibition serine could cause to the uptake of other amino acids was found to vary. Serine inhibited completely the uptake of glycine but a portion of uptake of long-chain amino acids was found to persist, even in the presence of high concentrations of serine. This is taken as evidence for the presence of an amino acid uptake mechanism having no affinity for serine. 4. The apparent affinities of neutral amino acids for the serino-inhibitable system (system 1) varied from about 0.5 mM (for long-chain amino acids) to about 3 mM (for short-chain amino acids). The total uptake of individual amino acids by system 1 was essentially similar when compared at an amino acid concentration of 1 mM. 5. The serine-resistant uptake of neutral amino acids (system 2) constituted up to two fifths of total uptake for long-chain amino acids, measured at amino acid concentrations of 1mM. The affinities of long-chain amino acids for system 2 is thought to be less than for system 1. Serine appears not to interact with system 2. 6. A second component to serine uptake was found to be related linearly to the concentration of serine in the medium. A similar component may contribute to the total uptake of phenylalanine. The possibility that such a component could arise as a space marker artifact is discussed. 7. An independent kinetic analysis of phenylalanine uptake by rabbit ileal mucosa showed that it could not be accounted for on the basis of a single entry system. However uptake could be described kinetically, assuming two systems of mediated entry to be present. The possible presence of a third non-saturable component to uptake does not affect these conclusions. 8. It is concluded that least two systems exist for the mediated entry of neutral amino acids into rabbit ileal mucosa. This fact should be taken into account in any future mechanistic interpretation of carrier-mediated amino acid transport in the small intestine.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bliss C. I., James A. T. Fitting the rectangular hyperbola. Biometrics. 1966 Sep;22(3):573–602. [PubMed] [Google Scholar]
- Christensen H. N., Handlogten M. E., Thomas E. L. Na plus-facilitated reactions of neutral amino acids with a cationic amino acid transport system. Proc Natl Acad Sci U S A. 1969 Jul;63(3):948–955. doi: 10.1073/pnas.63.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H. N. Some special kinetic problems of transport. Adv Enzymol Relat Areas Mol Biol. 1969;32:1–20. doi: 10.1002/9780470122778.ch1. [DOI] [PubMed] [Google Scholar]
- Daniels V. G., Dawson A. G., Newey H., Smyth D. H. Effect of carbon chain length and amino group position on neutral amino acid transport systems in rat small intestine. Biochim Biophys Acta. 1969 Apr;173(3):575–577. doi: 10.1016/0005-2736(69)90025-x. [DOI] [PubMed] [Google Scholar]
- Dugas M. C., Ramaswamy K., Crane R. K. An analysis of the D-glucose influx kinetics of in vitro hamster jejunum, based on considerations of the mass-transfer coefficient. Biochim Biophys Acta. 1975 Apr 8;382(4):576–589. doi: 10.1016/0005-2736(75)90224-2. [DOI] [PubMed] [Google Scholar]
- Erlij D., Smith M. W. Sodium uptake by frog skin and its modification by inhibitors of transepithelial sodium transport. J Physiol. 1973 Jan;228(1):221–239. doi: 10.1113/jphysiol.1973.sp010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar J. J., Curran P. F. Characteristics of the amino acid transport system in the mucosal border of rabbit ileum. J Gen Physiol. 1970 Dec;56(6):673–691. doi: 10.1085/jgp.56.6.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui Y., Christensen H. N. Discrimination of single transport systems. The Na plus-sensitive transport of neutral amino acids in the Ehrlich cell. J Gen Physiol. 1966 Sep;50(1):203–224. doi: 10.1085/jgp.50.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P. S., Smith M. W. Methionine transport by pig colonic mucosa measured during early post-natal development. J Physiol. 1976 Oct;262(1):151–168. doi: 10.1113/jphysiol.1976.sp011590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muflih I. W., Widdas W. F. Sugars and sugar derivatives which inhibit the short-circuit current of the everted small intestine of the rat. J Physiol. 1976 Dec;263(2):101–114. doi: 10.1113/jphysiol.1976.sp011623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWEY H., SMYTH D. H. THE TRANSFER SYSTEM FOR NEUTRAL AMINO ACIDS IN THE RAT SMALL INTESTINE. J Physiol. 1964 Mar;170:328–343. doi: 10.1113/jphysiol.1964.sp007334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellans H. N., Schultz S. G. Relations among transepithelial sodium transport, potassium exchange, and cell volume in rabbit ileum. J Gen Physiol. 1976 Oct;68(4):441–463. doi: 10.1085/jgp.68.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- Peterson S. C., Goldner A. M., Curran P. F. Glycine transport in rabbit ileum. Am J Physiol. 1970 Oct;219(4):1027–1032. doi: 10.1152/ajplegacy.1970.219.4.1027. [DOI] [PubMed] [Google Scholar]
- Preston R. L., Schaeffer J. F., Curran P. F. Structure-affinity relationships of substrates for the neutral amino acid transport system in rabbit ileum. J Gen Physiol. 1974 Oct;64(4):443–467. doi: 10.1085/jgp.64.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F., Chez R. A., Fuisz R. E. Alanine and sodium fluxes across mucosal border of rabbit ileum. J Gen Physiol. 1967 May;50(5):1241–1260. doi: 10.1085/jgp.50.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Markscheid-Kaspi L. Competitive interactions between L-alanine and L-phenylalanine in rabbit ileum. Biochim Biophys Acta. 1971 Sep 14;241(3):857–860. doi: 10.1016/0005-2736(71)90013-7. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Yu-Tu L., Strecker C. K. Influx of neutral amino acids across the brush border of rabbit ileum. Stereospecificity and the roles of the -amino and -carboxylate groups. Biochim Biophys Acta. 1972 Nov 2;288(2):367–379. doi: 10.1016/0005-2736(72)90258-1. [DOI] [PubMed] [Google Scholar]
- Smith M. W. Temperature adaptation in fish. Biochem Soc Symp. 1976;(41):43–60. [PubMed] [Google Scholar]
- Syme G., Levin R. J. The effects of hypothyroidism and fasting on electrogenic amino acid transfer: possible evidence for multiple neutral amino acid carrier systems in rat jejunum. Biochim Biophys Acta. 1977 Feb 4;464(3):620–628. doi: 10.1016/0005-2736(77)90036-0. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Dietschy J. M. The intestinal unstirred layer: its surface area and effect on active transport kinetics. Biochim Biophys Acta. 1974 Aug 21;363(1):112–126. doi: 10.1016/0005-2736(74)90010-8. [DOI] [PubMed] [Google Scholar]


