Abstract
Recently, we have shown that a vaccine consisting of a purified preparation of the Chlamydia trachomatis mouse pneumonitis (MoPn) major outer membrane protein (MOMP) and Freund's adjuvant can protect mice against a genital challenge. Here, we wanted to determine if CpG motifs could be used as an immune modulator to the MOMP to induce protection in mice against an intranasal (i.n.) challenge. One-week-old BALB/c mice were immunized intramuscularly and subcutaneously either once or three times at 2-week intervals with MOMP and CpG suspended in aluminum hydroxide (alum). Negative controls received ovalbumin, CpG, and alum. Positive controls were immunized i.n. with C. trachomatis MoPn elementary bodies (EB). Six weeks after the last immunization, mice were challenged i.n. with 104 inclusion-forming units (IFU) of the C. trachomatis MoPn serovar. Mice that received MOMP, CpG, and alum had a strong immune response, as shown by a high titer of serum antibodies to Chlamydia and significant lymphoproliferation of T-cells following stimulation with C. trachomatis EB. After the i.n. challenge mice immunized with MOMP, CpG, and alum showed significantly less body weight loss than the corresponding control mice immunized with ovalbumin, CpG, and alum. Ten days after the challenge the animals were euthanized, their lungs were weighed, and the numbers of IFU in the lungs were determined. The average weight of the lungs of the mice immunized with MOMP, CpG, and alum was significantly less than average weight of the lungs of the mice immunized with ovalbumin, CpG, and alum. Also, the average number of IFU recovered per mouse immunized with MOMP, CpG, and alum was significantly less than the average number of IFU per mouse detected in the mice inoculated with ovalbumin, CpG, and alum. In conclusion, our data show that CpG sequences can be used as an effective adjuvant with the C. trachomatis MoPn MOMP to elicit a protective immune response in mice against a chlamydial respiratory challenge.
Members of the genus Chlamydia cause infections throughout the animal kingdom (2, 3, 7, 27). This genus contains four species, and three of them, Chlamydia trachomatis, Chlamydia pneumoniae, and Chlamydia psittaci, can cause respiratory infections in humans (3, 26). Females that have a genital infection with C. trachomatis at the time of delivery transmit this organism to their infants 30 to 70% of the time and may have long-term sequelae, including chronic abdominal pain, ectopic pregnancy, and infertility (29, 35). In a newborn the clinical presentation may be an ocular infection and/or a pneumonia that frequently manifests in the first 6 months of life (3, 26). In certain parts of the world C. trachomatis is the most common cause of pneumonia in infants under 6 months old. In mature individuals C. trachomatis pneumonia is not common, but on the other hand, C. pneumoniae is one of the most common causes of adult pneumonia (3, 26). Respiratory infections due to C. psitacci usually occur in individuals working in close contact with infected wild birds (3, 26). Thus, the need to protect against Chlamydia infections is widespread throughout the world (2, 5, 8, 33).
The C. trachomatis mouse pneumonitis (MoPn) serovar was originally isolated from the lungs of mice inoculated with throat washings from humans with respiratory infections (17). This serovar was subsequently found to be a respiratory pathogen of mice, and thus, it is an ideal model to characterize potential vaccine candidates which could be used to prevent chlamydial respiratory infections (17, 24, 39). Optimally, a vaccine to protect against C. trachomatis infections should induce neutralizing antibodies on the mucosal surfaces while also stimulating a strong T-cell response (15, 16, 33, 36-38). More specifically, based on recent studies with several murine models it appears that a Th1 immune response is more effective than a Th2 response for protecting against a chlamydial challenge (9, 15, 16, 23). Nonreplicating antigens, including proteins such as the major outer membrane protein (MOMP), usually induce a predominant Th2 response. On the other hand, oligodeoxynucleotides containing immunostimulatory CpG motifs (CpG ODN) favor a Th1 response when they are administered to mice with a wide variety of antigens (10-13). Furthermore, CpG ODN have been found to induce significantly higher titers of antibodies against hepatitis B surface antigen, which was adsorbed to aluminum hydroxide (alum), significantly earlier in humans (H. L. Davis, C. L. Cooper, M. L. Morris, S. M. Elfer, D. W. Cameron, and J. Heathcote, Abstr. Third Annu. Conf. Vaccine Res., abstr, S25, p. 47, 2000). CpG ODN have broad effects, including stimulation of B cells to proliferate and secrete immunoglobulins and activation of macrophages, monocytes, and dendritic cells to produce cytokines and chemokines (6, 13, 34). We recently showed that a homogeneously purified preparation of the C. trachomatis MOMP administered with Freund's adjuvant can effectively protect mice against a genital challenge (21). Here, we wanted to determine the ability of this purified MOMP preparation to protect against an intranasal (i.n.) challenge. Furthermore, Freund's adjuvant is an adjuvant that cannot be utilized in humans, and thus, we decided to test an immunostimulant that potentially can be used in humans to protect against C. trachomatis infections. The results reported here show that CpG ODN are an effective adjuvant to the C. trachomatis MOMP for inducing a protective immune response in mice against an i.n. challenge.
MATERIALS AND METHODS
Organisms.
The C. trachomatis MoPn strain Nigg II was obtained from the American Type Culture Collection (Manassas, Va.). This organism was grown in HeLa 229 cells by using Eagle's minimal essential medium supplemented with 10% fetal calf serum. Elementary bodies (EB) were purified by using Renografin (Squibb, Princeton, N.J.) and were stored in sucrose-phosphate-glutamate (4).
Extraction and purification of the C. trachomatis MoPn MOMP.
The C. trachomatis MoPn strain that was grown in HeLa 229 cells was washed with 10 mM phosphate-buffered saline (PBS) (pH 7.4) and centrifuged, and the pellet was treated with DNase (21). Following centrifugation the pellet was resuspended in 0.2 M phosphate buffer (pH 5.5) containing 0.001 M EDTA and 0.001 M phenylmethylsulfonyl fluoride and extracted with 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) (Calbiochem-Novabiochem Corp., San Diego, Calif.) and Zwittergent 3-14 (Z3-14) (Calbiochem-Novabiochem Corp.) as previously described (21). The MOMP was recovered in the supernatant and was purified by using a hydroxylapatite column (1 by 35 cm) (4). The MOMP was refolded by dialysis against 0.1 M phosphate buffer (pH 7.8) containing 0.001 M EDTA, 0.002 M reduced glutathione, 0.001 M oxidized glutathione, and 0.05% Z3-14 at room temperature at a protein concentration of 30 to 150 μg/ml (21). The MOMP was concentrated and fixed with 20% glutaraldehyde for 2 min at room temperature, and subsequently 2 M glycine was added to stop the reaction. The preparation was concentrated with Centricon-10 filters and dialyzed against 0.02 M phosphate buffer (pH 7.4)-0.15 M NaCl-0.05% Z3-14 before inoculation (21).
Immunization protocol.
Groups of pregnant BALB/c (H2d) mice were purchased from Charles River Laboratories (Wilmington, Mass.). Following delivery 1-week-old male and female mice were simultaneously immunized intramuscularly (i.m.) and subcutaneously (s.c.) with (per animal per immunization) 10 μg of C. trachomatis MOMP in PBS (pH 7.4) containing 0.05% Z3-14, 10 or 50 μg of CpG, and 250 μg of alum (Alhydrogel 85; Superfos Biosectr a/s, Kvistgard, Denmark) (11-13, 20, 21). The mice that received 50 μg of CpG were immunized once, while the mice that received 10 μg of CpG were inoculated three times at 2-week intervals. We tested these two protocols based on the results previously reported by McCluskie and Davis (11, 12). As controls, mice were immunized with ovalbumin, CpG, and alum. CpG ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′) were provided by Coley Pharmaceutical Group (Wellesley, Mass.). As positive controls mice were immunized i.n. with 104 infection-forming units (IFU) of the C. trachomatis MoPn strain. All experiments were repeated. The protocols were approved by the Animal Care and Use Committee of the University of California, Irvine.
i.n. challenge.
Mice were challenged i.n. with 104 IFU of the C. trachomatis MoPn strain 6 weeks after the last immunization and were weighed daily for 10 days (19, 39, 40). For BALB/c mice the 50% lethal dose is 5 × 105 IFU/mouse (unpublished results). Mice were challenged 6 weeks after the last immunization in order to eliminate the possibility of developing nonspecific protection due the CpG (10, 11). At day 10 postchallenge the mice were euthanized, and the lungs were harvested and placed in 5 ml of sucrose-phosphate-glutamate (39, 40). The lungs were homogenized, and serial 10-fold dilutions were inoculated onto McCoy cells grown in 48-well tissue culture plates. The plates were incubated for 36 h at 37οC. Inclusions were stained with a pool of monoclonal antibodies prepared in our laboratory (19). This pool included monoclonal antibodies to the MOMP, the 60-kDa cysteine-rich protein, the 150-kDa putative outer membrane protein, and lipopolysaccharide of the C. trachomatis MoPn strain. The limit of detection was 50 IFU of the C. trachomatis MoPn strain per pair of lungs.
Immunoassays.
Blood samples were collected from the orbital plexus and from the heart on the day before the challenge, 41 and 69 days after the initial immunization for the mice inoculated once and three times, respectively. Chlamydia-specific antibodies were measured by an enzyme-linked immunosorbent assay (19). Flat-bottom 96-well plates were coated with C. trachomatis EB at a concentration of 10 μg per ml. A 1:2,000 dilution of goat anti-mouse pan immunoglobulin, immunoglobulin M (IgM), IgA, and IgG (Cappel, Aurora, Ohio) and a 1:3,000 dilution of goat anti-mouse IgG1, IgG2a, IgG2b, and IgG3 (Southern Biotechnology Associates, Birmingham, Ala.) were used to determine subclass- or isotype-specific antibodies.
The ability of serum to neutralize in vitro the infectivity of C. trachomatis MoPn EB was determined as follows (22). The C. trachomatis MoPn strain (104 IFU) was added to fourfold serial dilutions of serum made with 5% guinea pig serum in Ca2+- and Mg2+-free PBS. After incubation at 37°C for 45 min, the mixture was used to inoculate HeLa 229 cells by centrifugation. The cells were fixed 30 h after infection with methanol and stained as described above, and the number of IFU was determined. Neutralization was defined as ≥50% decrease in the number of IFU, as determined by using sera from animals inoculated with ovalbumin as a control.
For immunoblotting, components of C. trachomatis MoPn EB were resolved by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (28). Approximately 250 μg of purified EB was loaded on a 7.5-cm-wide slab gel. Following transfer to nitrocellulose membranes, the nonspecific sites were blocked with BLOTTO (bovine lacto transfer technique optimizer; 5% [wt/vol] nonfat dry milk, 2 mM CaCl2, 50 mM Tris-HCl [pH 8.0]), and serum samples diluted 1:100 were incubated overnight at 4°C (19, 21). Antibody binding was detected by using horseradish peroxidase-conjugated goat anti-mouse antibody developed with 0.01% hydrogen peroxide and 4-chloro-1-naphthol. Monoclonal antibody 40 to the C. trachomatis MoPn MOMP was used as a control.
A T-cell lymphoproliferative assay was performed by using splenocytes as previously described (19, 21). Briefly, T-enriched cells were counted, and 105 cells were placed in each well. UV-inactivated C. trachomatis MoPn EB were added at a ratio of EB to antigen-presenting cells of 10:1; the antigen-presenting cells were prepared by irradiating splenocytes with 3,300 rads. Some wells received medium alone as a negative control. Cell proliferation was measured by adding 1 μCi of [methyl-3H]thymidine per well. A mean count was obtained by using triplicate cultures.
Cytokine measurements.
Levels of gamma interferon (IFN-γ) (BD-Pharmingen, San Diego, Calif.) and interleukin-4 (IL-4) (Endogen, Cambridge, Mass.) were measured by using splenic T cells stimulated as described above and the tissue culture supernatants collected after 48 h of incubation (21).
Data analysis.
The two-tailed unpaired Student t test and the Mann-Whitney U test were employed to determine the significance of differences between the groups by using the Statview software program and a Macintosh computer (Apple Co., Cupertino, Calif.).
RESULTS
Characterization of the immunological response.
Results of the humoral immune response are shown in Table 1. High chlamydia-specific IgG and IgA antibody titers were observed in the groups of mice that were immunized by the i.m. and s.c. routes one or three times with MOMP, CpG, and alum. Both groups of experimental animals that received this type of immunization showed a balanced Th1 and Th2 response, as judged by the levels of IgG2a and IgG1 chlamydia-specific antibodies in the serum. Control mice immunized i.n. with C. trachomatis MoPn EB also had high levels of chlamydial IgG and IgA in the serum. This control group, on the other hand, had higher levels of IgG2a than of IgG1 in the serum, indicating a predominant Th1 response. Both control groups of animals that received ovalbumin, CpG, and alum had no detectable antibodies to Chlamydia.
TABLE 1.
Serum antibody titers of immunized mice the day before challenge with the C. trachomatis MoPn strain
| Antigen | Adjuvant | Immunization
|
Neutralizing titer | Anti-C. trachomatis MoPn ELISA titera
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Route | No. of times | IgM | IgA | IgG | IgG1 | IgG2a | IgG2b | IgG3 | |||
| MOMP | CpG + alum | i.m. + s.c. | 1 | 450 | <100 | 800 | 51,200 | 25,600 | 25,600 | 12,800 | 200 |
| Ovalbumin | CpG + alum | i.m. + s.c. | 1 | 0 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| MOMP | CpG + alum | i.m. + s.c. | 3 | 450 | <100 | 1,600 | 102,400 | 25,600 | 25,600 | 1,600 | 400 |
| Ovalbumin | CpG + alum | i.m. + s.c. | 3 | 0 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| C. trachomatis MoPn | i.n. | 1 | 1,250 | <100 | 3,200 | 25,600 | 3,200 | 25,600 | 12,800 | 3,200 | |
ELISA, enzyme-linked immunosorbent assay.
A neutralizing antibody titer of 450 was observed for both groups of mice immunized with MOMP, CpG, and alum (Table 1). Mice immunized i.n. with C. trachomatis EB had a neutralizing antibody titer of 1,250.
A Western blot prepared with serum collected the day before the i.n. challenge is shown in Fig. 1. Mice immunized with MOMP, CpG, and alum one or three times developed antibodies exclusively against MOMP, as shown by a band at approximately 40 kDa. Animals immunized i.n. with EB had predominantly antibodies to the MOMP, the 60-kDa cysteine-rich protein, and the 28-kDa protein. Control mice immunized with ovalbumin, CpG, and alum did not have antibodies that reacted with any of the chlamydial components.
FIG. 1.
Western blot of serum samples collected the day before the i.n. challenge. C. trachomatis MoPn EB were used as the antigen. Lane 1, molecular weight standards. Lanes 2 to 7, sera from mice immunized with MOMP, CpG, and alum once (lane 2); with ovalbumin, CpG, and alum once (lane 3); with MOMP, CpG, and alum three times (lane 4); with ovalbumin, CpG, and alum three times (lane 5); with C. trachomatis MoPn EB (lane 6); or with saline (lane 7). Lane 8, control monoclonal antibody 40 to the C. trachomatis MoPn MOMP.
The results of measurement of the cell-mediated immune response are shown in Table 2. T-lymphocytes from animals immunized with MOMP, CpG, and alum one or three times by the i.m. and s.c. routes showed a significant lymphoproliferative response when they were stimulated with EB compared with the response of the control mice immunized with ovalbumin, CpG, and alum (P < 0.05). Control BALB/c mice immunized i.n. with EB also showed a strong lymphoproliferative response to chlamydial EB. The levels of IFN-γ in supernatants of splenocytes stimulated with EB were significantly higher than the levels of IL-4 for the mice immunized with MOMP, CpG, and alum and for the control animals inoculated i.n. with EB. Overall, the immune response in the animals immunized three times was stronger, as determined by the high proliferative response and the levels of IFN-γ production, than the immune response in the animals immunized only once.
TABLE 2.
T-cell responses of immunized mice the day before challenge with the C. trachomatis MoPn straina
| Antigen | Adjuvant | Immunization
|
T-cell proliferative response (103 cpm) to:
|
In vitro cytokine production (pg/ml)
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| IFN-γ
|
IL-4
|
||||||||
| Route | No. of times | EB | Medium | EB | Medium | EB | Medium | ||
| MOMP | CpG + alum | i.m. + s.c. | 1 | 6.5 ± 1.8b | 0.09 ± 0.01 | 555 ± 31b | <30 | 1.4 ± 0.7 | <0.1 |
| Ovalbumin | CpG + alum | i.m. + s.c. | 1 | 0.3 ± 0.2 | 0.1 ± 0.02 | <30 | <30 | <0.1 | <0.1 |
| C. trachomatis | i.n. | 1 | 6.9 ± 1.1b | 0.1 ± 0.04 | 4,275 ± 28b | <30 | 5.1 ± 0.2b | <0.1 | |
| MOMP | CpG + alum | i.m. + s.c. | 3 | 26.1 ± 4.1b | 1.5 ± 0.2 | 1,306 ± 85b | <30 | 4.4 ± 1.3 | <0.1 |
| Ovalbumin | CpG + alum | i.m. + s.c. | 3 | 0.4 ± 0.1 | 0.3 ± 0.08 | 39 ± 24 | <30 | 0.5 ± 0.7 | <0.1 |
| C. trachomatis | i.n. | 1 | 6.1 ± 0.8b | 0.2 ± 0.1 | 4,009 ± 137b | <30 | 5.1 ± 1.2b | <0.1 | |
The values are means ± standard errors for triplicate cultures. The data are from one of the experiments representative of duplicate experiments. UV-inactivated C. trachomatis MoPn EB were added at a ratio of EB to T cells of 10:1.
P < 0.05 as determined by the Student t test for the comparison with the corresponding ovalbumin-immunized group.
Changes in body weight following the i.n. challenge.
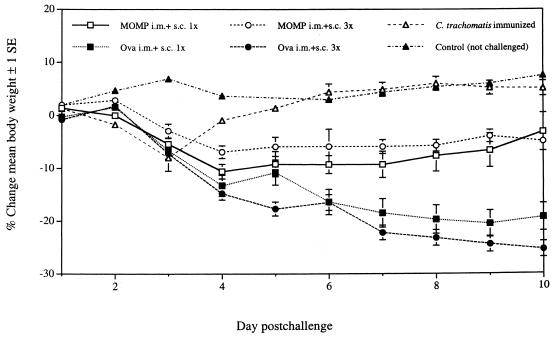
The changes in mean body weight of the mice following the i.n. challenge are shown in Fig. 2. A group of mice that was not challenged served as a control for possible changes in body weight in normal animals. As expected, no significant changes in body weight were observed for this control group over the 10 days of observation. Mice immunized once or three times with MOMP, CpG, and alum lost weight for the first 4 days and then slowly started to recover. By 10 days after the i.n. challenge the body weights of both groups were approximately only 3 to 5% below the initial body weights. On the other hand, animals immunized with ovalbumin, CpG, and alum lost body weight during the 10 days of observation, and the final body weights were approximately 20 to 25% below the initial body weights (P < 0.05). The mice that were vaccinated i.n. with EB showed a significant drop in body weight for 3 days after the i.n. challenge but then quickly returned to the expected weight.
FIG. 2.
Changes in mean body weights of the six groups of mice following i.n. challenge. Ova, ovalbumin.
Characterization of the lungs.
Ten days after the i.n. challenge mice were euthanized, and their lungs were weighed and cultured. As shown in Table 3, the lungs of the BALB/c mice immunized once i.m. and s.c. with MOMP, CpG, and alum weighed 0.24 g, while the lungs of the mice immunized with ovalbumin, CpG, and alum weighed 0.30 g (P = 0.01). The yields of chlamydial IFU were also significantly different for the two groups (2.3 × 106 versus 43.2 × 106 IFU; P = 0.01). The weight of the lungs of the mice inoculated with EB was similar to the weight of the lungs of the animals immunized with MOMP and significantly different from the weight of the lungs of the animals that were inoculated with ovalbumin, CpG, and alum (P < 0.0001). In the control group of mice the number of IFU was below the level of detection.
TABLE 3.
Recovery of C. trachomatis MoPn IFU from the lungs of mice following i.n. challenge
| No. of mice/group | Antigen | Adjuvant | Immunization
|
Wt of lungs (g)a | No. of IFU recovered from lungs (106)a | |
|---|---|---|---|---|---|---|
| Route | No. of times | |||||
| 13 | MOMP | CpG + alum | i.m. + s.c. | 1 | 0.24 ± 0.02b | 2.3 ± 1.5c |
| 14 | Ovalbumin | CpG + alum | i.m. + s.c. | 1 | 0.30 ± 0.02 | 43.2 ± 17.9 |
| 9 | C. trachomatis MoPn | i.n. | 1 | 0.23 ± 0.01b | <0.00005c | |
| 13 | MOMP | CpG + alum | i.m. + s.c. | 3 | 0.23 ± 0.02b | 0.8 ± 0.3c |
| 13 | Ovalbumin | CpG + alum | i.m. + s.c. | 3 | 0.38 ± 0.01 | 241.8 ± 90.8 |
| 11 | C. trachomatis MoPn | i.n. | 1 | 0.26 ± 0.01b | 0.001 ± 0.0001c | |
The values are means ± standard errors.
P < 0.05 as determined by the Student t test for the comparison with the corresponding ovalbumin-immunized group.
P < 0.05 as determined by the Mann-Whitney U test for the comparison with the corresponding ovalbumin-immunized group.
Significant differences were also observed between the groups of mice immunized three times. Animals immunized three times exhibited a higher T-cell proliferative response, a higher level of IFN-γ production, and better protection than the mice immunized only once. The mean weight of the lungs of the BALB/c mice immunized with MOMP, CpG, and alum was 0.23 g, while the lungs of the mice immunized with ovalbumin, CpG, and alum weighed 0.38 g (P < 0.0001). The yield of Chlamydia was 0.8 × 106 IFU from the lungs of the animals immunized with MOMP, CpG, and alum, while we recovered 241.8 × 106 IFU from the controls (P < 0.0001). As expected, the BALB/c mice inoculated i.n. with MoPn EB were well protected, as shown by the weight of the lungs (P < 0.001) and the yield of IFU (P < 0.0001) compared with the values for the control group of animals immunized with ovalbumin plus CpG plus alum.
DISCUSSION
Here we show that a preparation of the chlamydial MOMP administered with CpG ODN plus alum as an immune modulator can induce protection against a C. trachomatis i.n. challenge. To our knowledge, this is the first time that a homogeneously purified antigen of C. trachomatis, directly extracted from this organism, has been shown to induce significant protection against a respiratory challenge when it is combined with an adjuvant that potentially can be used in humans. The protection that we obtained by immunizing mice with MOMP, CpG, and alum followed by two boost inoculations with the same preparation paralleled that observed after i.n. immunization with viable organisms. These results are very encouraging since up to this point, vaccination with viable EB has been considered to be the most efficient way of obtaining protection against a C. trachomatis challenge (8, 19, 40).
Over the last several decades multiple laboratories have attempted to develop a vaccine against Chlamydia infections (8, 18, 24). Some of the first efforts were focused on controlling trachoma, and whole viable or inactivated organisms were used as the antigen to immunize humans and monkeys (8). The recognition over the last three or four decades of the high prevalence of chlamydial respiratory and genital infections has resulted in a new impetus for developing a vaccine against this pathogen (8, 18, 21, 25, 33).
Although no vaccination protocols were implemented as a result of the initial efforts to control trachoma, some important conclusions were obtained from those trials (8). Protection against a chlamydial challenge was found to be serovar specific and short lived. Furthermore, in individuals that were only partially protected reexposure resulted in clinical disease that was more severe than the disease occurring in nonvaccinated controls. The fact that the initial trials with whole organisms resulted in some cases of what appeared to be a hypersensitivity reaction prompted attempts to develop subunit vaccines (8, 14). As a result of its ability to induce a strong immune response, the MOMP was identified as a promising candidate (1, 2, 5, 24, 30, 33). However, multiple attempts to immunize different animals with MOMP extracted from the organism or recombinant preparations gave disappointing results (20, 31-33). On the other hand, Zhang et al. in 1997 reported that DNA vaccination with the MOMP gene was able to induce a protective immune response against an i.n. challenge (39). The protection, however, was limited and varied from experiment to experiment (39, 40). Pal et al. also attempted to induce protection against a genital challenge using a DNA vaccine with the MOMP gene but obtained negative results (18). Recently, Zhang et al. (40) vaccinated mice with the MOMP plasmid and then boosted with immune-stimulating complexes and the MOMP of Chlamydia. Using this approach, they were able to obtained significant protection against an i.n. challenge.
The apparent inefficiency of the MOMP preparations for inducing a strong protective immune response, in contrast to the efficiency of vaccination with the whole organism or the outer membrane, was considered to be due to the loss of conformational epitopes present in MOMP in the intact organism (20, 33, 40). In support of this hypothesis, Pal et al. recently reported that a refolded preparation of MOMP, in which Freund's adjuvant was used as an adjuvant, was able to induce significant protection against a genital challenge (21). Furthermore, disruption of the structural conformation by sonication of the refolded MOMP preparation resulted in a decrease in protective activity (21). Freund's adjuvant, however, cannot be used in humans, and thus, here we tested the ability of CpG and alum, compounds already tested in humans, to act as immune modulators to the refolded C. trachomatis MOMP preparation. Our results are very encouraging and support the findings, reported for other systems, which indicate that the adjuvant effect of CpG ODN and alum in adult and neonatal mice is strong (10-13). CpG ODN stimulate the production of IL-6, IL-12, and IFN-γ with a strong Th1 component (10-13, 34). Alum, on the other hand, favors a Th2 response (34). Thus, it is not surprising that we obtained a mixed Th1-Th2 response, as shown by the levels of IgG1 and IgG2a, although higher levels of IFN-γ than of IL-4 were detected in supernatants from splenocytes following stimulation with EB.
In conclusion, we have shown that a homogeneously purified preparation of MOMP administered with CpG plus alum can induce significant protection in mice against a C. trachomatis respiratory challenge. Before a similar protocol can be implemented in humans, several issues will have to be addressed. Production of enough MOMP by extraction from tissue culture-grown C. trachomatis is costly. On the other hand, preparation of a recombinant protein should solve this problem, but refolding of the MOMP in the protective conformation may be a significant challenge. Also, our results were obtained by using the MoPn serovar, which, although having a high degree of DNA sequence homology to the human serovars, may have some unique properties. Thus, testing of MOMP preparations purified from human serovars by using a monkey model will have to be done to test the validity of this approach. Finally, it will be necessary to carry out appropriate safety and efficacy evaluations of the combination of MOMP plus CpG plus alum to support a human clinical study. In spite of the limitations, our results and those previously reported indicating that this MOMP preparation can also induce protection against a genital challenge have provided very encouraging evidence of the potential for using this protein as a vaccine candidate in humans.
Acknowledgments
This work was supported by Public Health Service grant AI-32248 from the National Institute of Allergy and Infectious Diseases.
Editor: R. N. Moore
REFERENCES
- 1.Baehr, W., Y. X. Zhang, T. Joseph, H. Su, F. E. Nano, K. D. E. Everett, and H. D. Caldwell. 1988. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc. Natl. Acad. Sci. USA 85:4000-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beagley, K. W., and P. Timms. 2000. Chlamydia trachomatis infection: incidence, health costs and prospects for vaccine development. J. Reprod. Immunol. 48:47-68. [DOI] [PubMed] [Google Scholar]
- 3.Brunham, R. C., and G. McClarty. 2000. Chlamydia, p. 339-367. In L. R. Stanberry and D. I. Bernstein (ed.), Sexually transmitted diseases: vaccines, prevention and control. Academic Press, London, United Kingdom.
- 4.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Maza, M. A., and L. M. de la Maza. 1995. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine 13:119-127. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh, D. K., M. A. Misukonis, C. Reich, D. Pisetsky, and J. B. Weinberg. 2001. Host response to infection: the role of CpG DNA in induction of cyclooxygenase 2 and nitric oxide synthase 2 in murine macrophages. Infect. Immun. 69:7703-7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grayston, J. T., and S. P. Wang. 1975. New knowledge of chlamydiae and the diseases they cause. J. Infect. Dis. 132:87-105. [DOI] [PubMed] [Google Scholar]
- 8.Grayston, J. T., and S. P. Wang. 1978. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex. Transm. Dis. 5:73-77. [DOI] [PubMed] [Google Scholar]
- 9.Igietseme, J. U., K. H. Ramsey, D. M. Magee, D. M. Williams, T. J. Kincy, and R. G. Rank. 1993. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific Th1 lymphocyte clone. Reg. Immunol. 5:317-324. [PubMed] [Google Scholar]
- 10.Krieg, A. M. 2001. Immune effects and mechanims of action of CpG motifs. Vaccine 19:618-622. [DOI] [PubMed] [Google Scholar]
- 11.McCluskie, M. J., and H. L. Davis. 2000. CpG DNA as mucosal adjuvant. Vaccine 18:231-237. [DOI] [PubMed] [Google Scholar]
- 12.McCluskie, M. J., and H. L. Davis. 2001. Oral, intrarectal and intranasal immunization using CpG and non-CpG oligodeoxynucleotides as adjuvants. Vaccine 19:413-422. [DOI] [PubMed] [Google Scholar]
- 13.McCluskie, M. J., R. D. Weeratna, and H. L. Davis. 2001. The potential of oligodeoxynucleotides as mucosal and parenteral adjuvants. Vaccine 19:2657-2660. [DOI] [PubMed] [Google Scholar]
- 14.Morrison, R. P., R. J. Belland, K. Lyng, and H. D. Caldwell. 1989. Chlamydial disease pathogenesis. The 57-kDa chlamydial hypersensitivity antigen is a stress response protein. J. Exp. Med. 170:113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison, S. G., and R. P. Morrison. 2001. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect. Immun. 69:2643-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 68:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nigg, C. 1942. An unidentified virus which produces pneumonia and systemic infection in mice. Science 99:49-50. [DOI] [PubMed] [Google Scholar]
- 18.Pal, S., K. M. Barnhart, Q. Wei, A. M. Abai, E. M. Peterson, and L. M. de la Maza. 1999. Vaccination of mice with DNA plasmids coding for the Chlamydia trachomatis major outer membrane protein elicits an immune response but fails to protect against a genital challenge. Vaccine 17:459-465. [DOI] [PubMed] [Google Scholar]
- 19.Pal, S., T. J. Fielder, E. M. Peterson, and L. M. de la Maza. 1994. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 62:3354-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 1997. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect. Immun. 65:3361-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 2001. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect. Immun. 69:6240-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson, E. M., G. Zhong, E. Carlson, and L. M. de la Maza. 1988. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect. Immun. 56:885-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsey, K. H., L. S. F. Soderberg, and R. G. Rank. 1988. Resolution of chamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect. Immun. 56:1320-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rank, R. G. 1999. Models of immunity, p. 239-296. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, D.C.
- 25.Rank, R. G., H. J. White, and A. L. Barron. 1979. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect. Immun. 26:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schachter, J. 1999. Infection and disease epidemiology, p. 139-170. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, D.C.
- 27.Schachter, J., and C. Dawson. 1978. Human chlamydial infections. PSG Publishing Company, Inc., Littleton, Mass.
- 28.Schagger, H., and G. Von Jagow. 1987. Tricine-sodium dodecyl sulphate polyacrylamide gel electrophoresis for the separation of protein range 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 29.Stamm, W. E. 1999. Chlamydia trachomatis infections of the adult, p. 407-422. In K. K. Holmes et al. (ed.), Sexually transmitted diseases. McGraw Hill, New York, N.Y.
- 30.Stephens, R. S., R. Sanchez-Pescador, E. A. Wagar, C. Inouye, and M. S. Urdea. 1987. Diversity of Chlamydia trachomatis major outer membrane protein genes. J. Bacteriol. 169:3879-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor, H. R., J. Whittum-Hudson, J. Schachter, H. D. Caldwell, and R. A. Prendergast. 1988. Oral immunization with chlamydial major outer membrane protein (MOMP). Investig. Ophthalmol. Vis. Sci. 29:1847-1853. [PubMed] [Google Scholar]
- 32.Tuffrey, M., F. Alexander, W. Conlan, C. Woods, and M. Ward. 1992. Heterotypic protection of mice against chlamydial salpingitis and colonization of the lower genital tract with a human serovar F isolate of Chlamydia trachomatis by prior immunization with recombinant serovar L1 major outer-membrane protein. J. Gen. Microbiol. 148:1707-1715. [DOI] [PubMed] [Google Scholar]
- 33.Ward, M. E. 1992. Chlamydial vaccines—future trends. J. Infect. 25:11-26. [DOI] [PubMed] [Google Scholar]
- 34.Weeratna, R. D., McCluskie, M. J., Xu, Y., and H. L. Davis. 2000. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine 18:1755-1762. [DOI] [PubMed] [Google Scholar]
- 35.Westrom, L., R. Joesoef, G. Reynolds, A. Hagdu, and S. E. Thompson. 1992. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopy. Sex. Transm. Dis. 19:185-192. [PubMed] [Google Scholar]
- 36.Williams, D. M., J. Schachter, B. Grubbs, and C. V. Sumaya. 1982. The role of antibody in host defense against the agent of mouse pneumonitis. J. Infect. Dis. 145:200-205. [DOI] [PubMed] [Google Scholar]
- 37.Williams, D. M., J. Schachter, J. C. Coalson, and B. Grubbs. 1982. Cellular immunity to the mouse pneumonitis agent. J. Infect. Dis. 149:630-639. [DOI] [PubMed] [Google Scholar]
- 38.Williams, D. M., J. Schachter, M. H. Weiner, and B. Grubbs. 1984. Antibody in host defense against mouse pneumonitis agent (murine Chlamydia trachomatis). Infect. Immun. 45:64-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, D. J., J. Berry, C. Shen, G. McClarty, and R. C. Brunham. 1997. DNA vaccination with the major outer-membrane protein gene induces immunity to Chlamydia trachomatis (mouse pneumonitis infection). J. Infect. Dis. 176:1035-1040. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, D.-J., X. Yang, C. Shen, H. Lu, A. Murdin, and R. C. Brunham. 2000. Priming with Chlamydia trachomatis major outer membrane protein (MOMP) DNA followed by MOMP immune-stimulating complex boosting enhances protection and is associated with increased immunoglobulin A and Th1 cellular immune responses. Infect. Immun. 68:3074-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]