Abstract
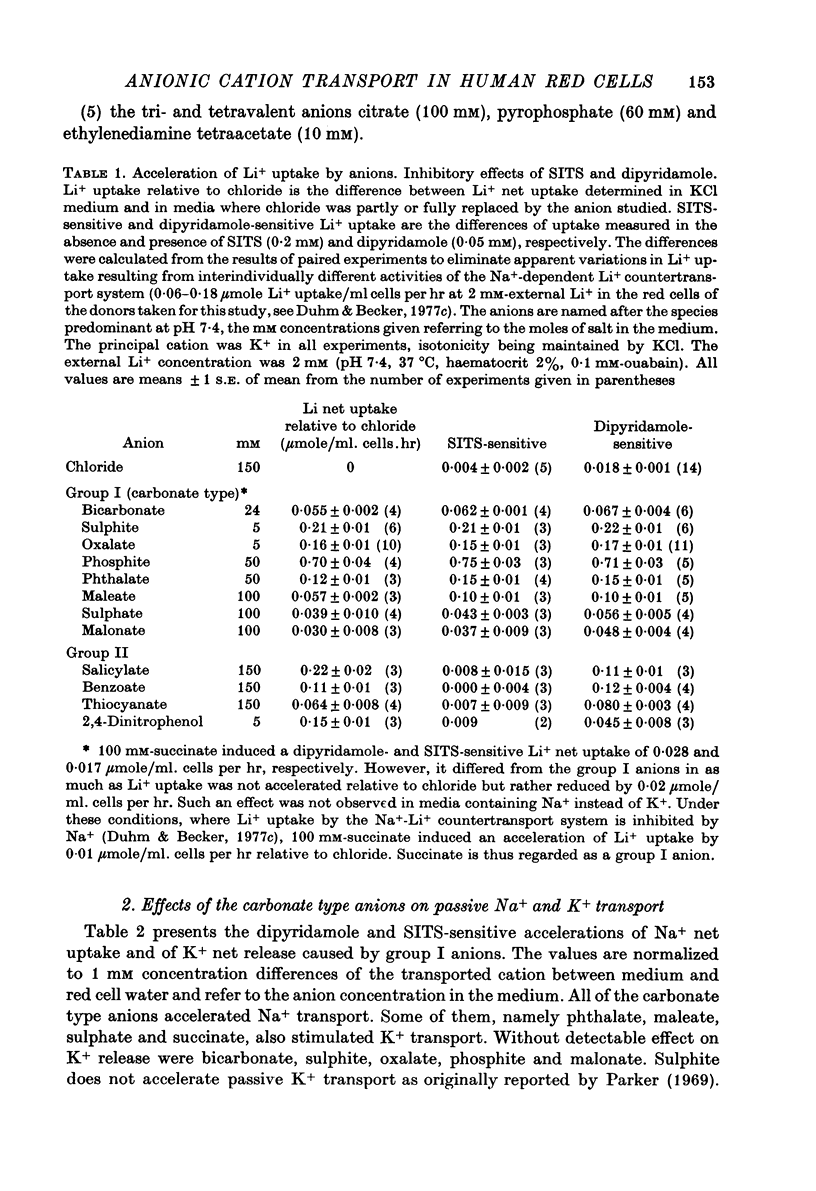
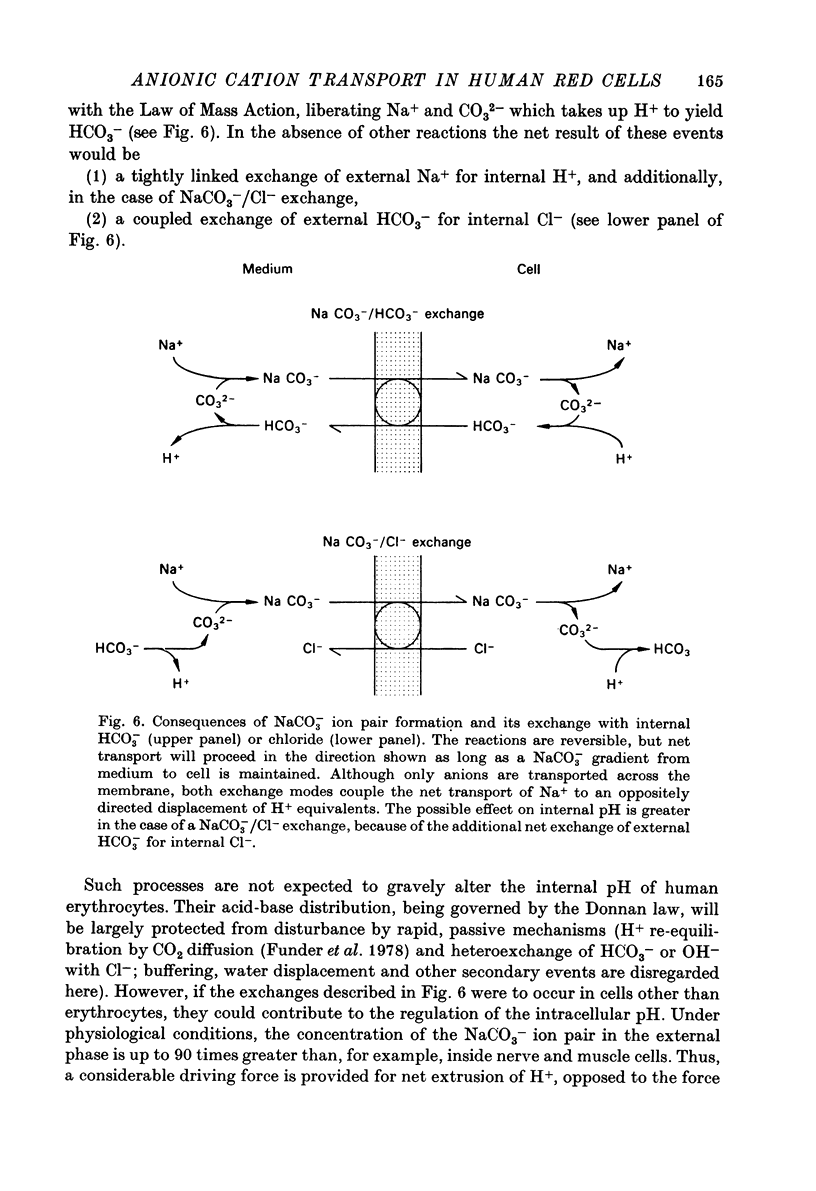
1. The passive net transport of Li+ and Na+ across the human red cell membrane was accelerated by the divalent anions carbonate, sulphite, oxalate, phosphite and malonate. Phthalate, maleate, sulphate and succinate were found additionally to stimulate downhill transport of K+. Marked differences in anion efficacy and selectivity were observed. 2. The effects of these 'carbonate type' anions were reversible and fully blocked by SITS, dipyridamole and other inhibitors of anion transfer. 3. Cation transport acceleration induced by the monovalent anions salicylate, benzoate, thiocyanate and 2,4-dinitrophenol were inhibited by dipyridamole, but not affected by SITS. A great number of mono- and polyvalent anions were without detectable influence on Li+ transport. 4. Li+ net uptake induced by oxalate exhibited a pH dependence similar to that reported for halide self exchange. 5. Transport acceleration by carbonate type anions displayed a linear, 1:1 dependence on the concentrations of both the anion and the cation and was symmetric with respect to the two sides of the membrane. 6. It is concluded that the divalent carbonate type anions form singly charged, negative 1:1 ion pairs with the respective alkali metal cations, the ion pairs traversing the red cell membrane via the anion exchange pathway. This concept of anionic formation of some of the ion pairs considered. The relative efficacies and cation selectivities of polyvalent anions can largely be explained on the basis of electrostatic interactions governing ion pair formation. However, the chelating properties, structural flexibility, polarizability of the anions and the accessibility of the ion pairs to the anion exchange pathway need also be considered. 7. An exchange of NaCO-3 ion pairs for internal HCO-3 or Cl- is discussed as a possible mode of cellular pH regulation.
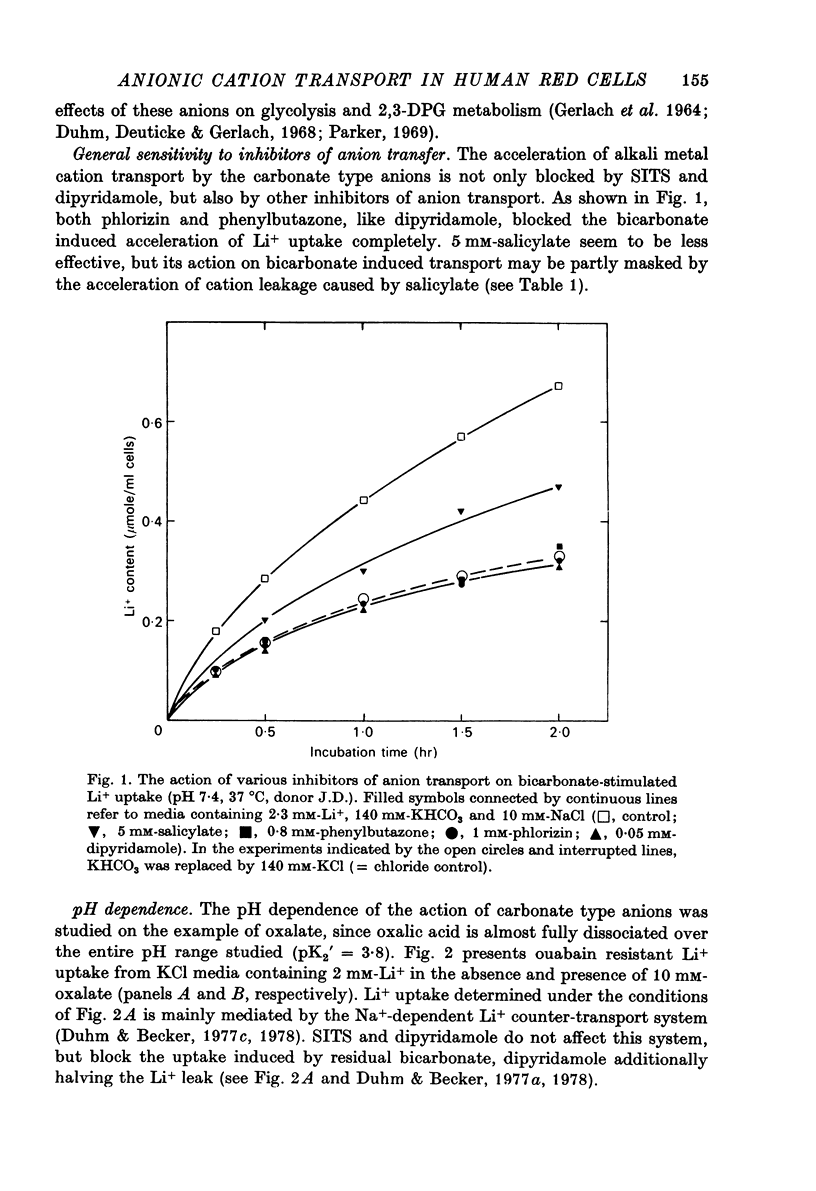
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN O. S. The first dissociation exponent of carbonic acid as a function of pH. Scand J Clin Lab Invest. 1962;14:587–597. [PubMed] [Google Scholar]
- Astrup J. The effect of hypokalaemia and of digoxin therapy on red cell sodium and potassium content. Some clinical aspects. Scand J Clin Lab Invest. 1974 Feb;33(1):11–16. doi: 10.3109/00365517409114191. [DOI] [PubMed] [Google Scholar]
- Aubert L., Motais R. Molecular features of organic anion permeablity in ox red blood cell. J Physiol. 1975 Mar;246(1):159–179. doi: 10.1113/jphysiol.1975.sp010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahm J. Temperature-dependent changes of chloride transport kinetics in human red cells. J Gen Physiol. 1977 Sep;70(3):283–306. doi: 10.1085/jgp.70.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Dalmark M., Wieth J. O. Temperature dependence of chloride, bromide, iodide, thiocyanate and salicylate transport in human red cells. J Physiol. 1972 Aug;224(3):583–610. doi: 10.1113/jphysiol.1972.sp009914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Wright E. M. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- Duhm J., Becker B. F. Studies on the lithium transport across the red cell membrane. I.V. Interindividual variations in the Na+-dependent Li+ countertransport system of human erythrocytes. Pflugers Arch. 1977 Sep 16;370(3):211–219. doi: 10.1007/BF00585529. [DOI] [PubMed] [Google Scholar]
- Duhm J., Becker B. F. Studies on the lithium transport across the red cell membrane. II. Characterization of ouabain-sensitive and ouabain-insensitive Li+ transport. Effects of bicarbonate and dipyridamole. Pflugers Arch. 1977 Jan 17;367(3):211–219. doi: 10.1007/BF00581357. [DOI] [PubMed] [Google Scholar]
- Duhm J., Becker B. F. Studies on the lithium transport across the red cell membrane. III. Factors contributing to the intraindividual variability of the in vitro Li+ distribution across the human red cell membrane. Pflugers Arch. 1977 Apr 25;368(3):203–208. doi: 10.1007/BF00585197. [DOI] [PubMed] [Google Scholar]
- Duhm J., Deuticke B., Gerlach E. Metabolism of 2,3-diphosphoglycerate and glycolysis in humna red blood cells under the infleucne of dipyridamole and inorganic sulfur compounds. Biochim Biophys Acta. 1968 Dec 23;170(2):452–454. doi: 10.1016/0304-4165(68)90033-0. [DOI] [PubMed] [Google Scholar]
- Eisenman G. The molecular basis for ion selectivity and its possible bearing on the neurobiology of lithium. Neurosci Res Program Bull. 1976 Apr;14(2):154–161. [PubMed] [Google Scholar]
- Frausto da Silva J. J., Williams R. J. Possible mechanism for the biological action of lithium. Nature. 1976 Sep 16;263(5574):237–239. doi: 10.1038/263237a0. [DOI] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Combined effects of digitalis therapy and of plasma bicarbonate on human red cell socium and potassium. Scand J Clin Lab Invest. 1974 Oct;34(2):153–160. [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Human red cell sodium and potassium in metabolic alkalosis. Scand J Clin Lab Invest. 1974 Sep;34(1):49–59. doi: 10.3109/00365517409061821. [DOI] [PubMed] [Google Scholar]
- GERLACH E., DEUTICKE B., DUHM J. PHOSPHAT-PERMEABILITAET UND PHOSPHAT-STOFFWECHSEL MENSCHLICHER ERYTHROCYTEN UND MOEGLICHKEITEN IHRER EXPERIMENTELLEN BEEINFLUSSUNG. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jul 30;280:243–274. [PubMed] [Google Scholar]
- GIEBEL O., PASSOW H. [The permeability of erythrocyte membranes for organic anions. On the problem of diffusion through the pores]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;271:378–388. [PubMed] [Google Scholar]
- Gros G., Forster R. E., Lin L. The carbamate reaction of glycylglycine, plasma, and tissue extracts evaluated by a pH stopped flow apparatus. J Biol Chem. 1976 Jul 25;251(14):4398–4407. [PubMed] [Google Scholar]
- Gunn R. B., Tosteson D. C. The effect of 2,4,6-trinitro-m-cresol on cation and anion transport in sheep red blood cells. J Gen Physiol. 1971 May;57(5):593–609. doi: 10.1085/jgp.57.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haest C. W., Kamp D., Plasa G., Deuticke B. Intra- and intermolecular cross-linking of membrane proteins in intact erythrocytes and ghosts by SH-oxidizing agents. Biochim Biophys Acta. 1977 Sep 5;469(2):226–230. doi: 10.1016/0005-2736(77)90186-9. [DOI] [PubMed] [Google Scholar]
- Hunter M. J. Human erythrocyte anion permeabilities measured under conditions of net charge transfer. J Physiol. 1977 Jun;268(1):35–49. doi: 10.1113/jphysiol.1977.sp011845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H., Scorah K., Fasold H., Passow H. Sidedness of the inhibitory action of disulfonic acids on chloride equilibrium exchange and net transport across the human erythrocyte membrane. FEBS Lett. 1976 Feb 15;62(2):182–185. doi: 10.1016/0014-5793(76)80048-8. [DOI] [PubMed] [Google Scholar]
- Knauf P. A., Fuhrmann G. F., Rothstein S., Rothstein A. The relationship between anion exchange and net anion flow across the human red blood cell membrane. J Gen Physiol. 1977 Mar;69(3):363–386. doi: 10.1085/jgp.69.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf P. A., Rothstein A. Chemical modification of membranes. I. Effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell. J Gen Physiol. 1971 Aug;58(2):190–210. doi: 10.1085/jgp.58.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. L., Rector F. C., Jr, Seldin D. W. The effects of chronic hypokalaemia, hyponatraemia, and acid-base alterations on erythrocyte sodium transport. Clin Sci. 1972 Aug;43(2):251–263. doi: 10.1042/cs0430251. [DOI] [PubMed] [Google Scholar]
- Needle M. A., Shapiro W., Viswanathan V., Semar M. Relation of the extracellular (bicarbonate)-(chloride) ratio to erythrocyte sodium content: a possible new control system. Clin Sci. 1972 Sep;43(3):311–318. doi: 10.1042/cs0430311. [DOI] [PubMed] [Google Scholar]
- Parker J. C. Influence of 2,3-diphosphoglycerate metabolism on sodium-potassium permeability in human red blood cells: studies with bisulfite and other redox agents. J Clin Invest. 1969 Jan;48(1):117–125. doi: 10.1172/JCI105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. C., George P., Rutman R. J. Thermodynamic studies of the formation and ionization of the magnesium(II) complexes of ADP and ATP over the pH range 5 to 9. J Am Chem Soc. 1966 Jun 20;88(12):2631–2640. doi: 10.1021/ja00964a002. [DOI] [PubMed] [Google Scholar]
- SCHUBERT J. Measurement of complex ion stability by the use of ion exchange resins. Methods Biochem Anal. 1956;3:247–263. doi: 10.1002/9780470110195.ch8. [DOI] [PubMed] [Google Scholar]
- Schnell K. F. On the mechanism of inhibition of the sulfate transfer across the human erythrocyte membrane. Biochim Biophys Acta. 1972 Sep 1;282(1):265–276. doi: 10.1016/0005-2736(72)90333-1. [DOI] [PubMed] [Google Scholar]
- TSEN C. C., TAPPEL A. L. Catalytic oxidation of glutathione and other sulfhydryl compounds by selenite. J Biol Chem. 1958 Nov;233(5):1230–1232. [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieth J. O. Effect of some monovalent anions on chloride and sulphate permeability of human red cells. J Physiol. 1970 May;207(3):581–609. doi: 10.1113/jphysiol.1970.sp009082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieth J. O. Effects of bicarbonate and thiocyanate on fluxes of Na and K, and on glucose metabolism of actively transporting human red cells. Acta Physiol Scand. 1969 Mar;75(3):313–329. doi: 10.1111/j.1748-1716.1969.tb04384.x. [DOI] [PubMed] [Google Scholar]
- Wieth J. O. Effects of monovalent cations on sodium permeability of human red cells. Acta Physiol Scand. 1970 May;79(1):76–87. doi: 10.1111/j.1748-1716.1970.tb04703.x. [DOI] [PubMed] [Google Scholar]
- Wieth J. O., Funder J. An effect of anoins on transfer of sodium through the human red cell membrane. Scand J Clin Lab Invest. 1965;17(4):399–400. doi: 10.3109/00365516509077069. [DOI] [PubMed] [Google Scholar]
- Wieth J. O. Paradoxical temperature dependence of sodium and potassium fluxes in human red cells. J Physiol. 1970 May;207(3):563–580. doi: 10.1113/jphysiol.1970.sp009081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]
- Zaki L., Fasold H., Schuhmann B., Passow H. Chemical modification of membrane proteins in relation to inhibition of anion exchange in human red blood cells. J Cell Physiol. 1975 Dec;86(3 Pt 1):471–494. doi: 10.1002/jcp.1040860305. [DOI] [PubMed] [Google Scholar]


