Abstract
1. Cortical slices from kidneys of adult rats were incubated for 1 and 2 hr at 25 °C in oxygenated solutions containing 5 mM-K, 2·5 mM-Ca and Mg, 0·007 M-phosphate buffer (pH 7·4) and 5 mM-glucose, together with 1, 2, 4 and 6% polyethylene glycol (PEG 6000) and 46, 92, 123, 154 and 231 mM-NaCl.
2. There were no significant differences in water content between slices incubated for 1 and for 2 hr.
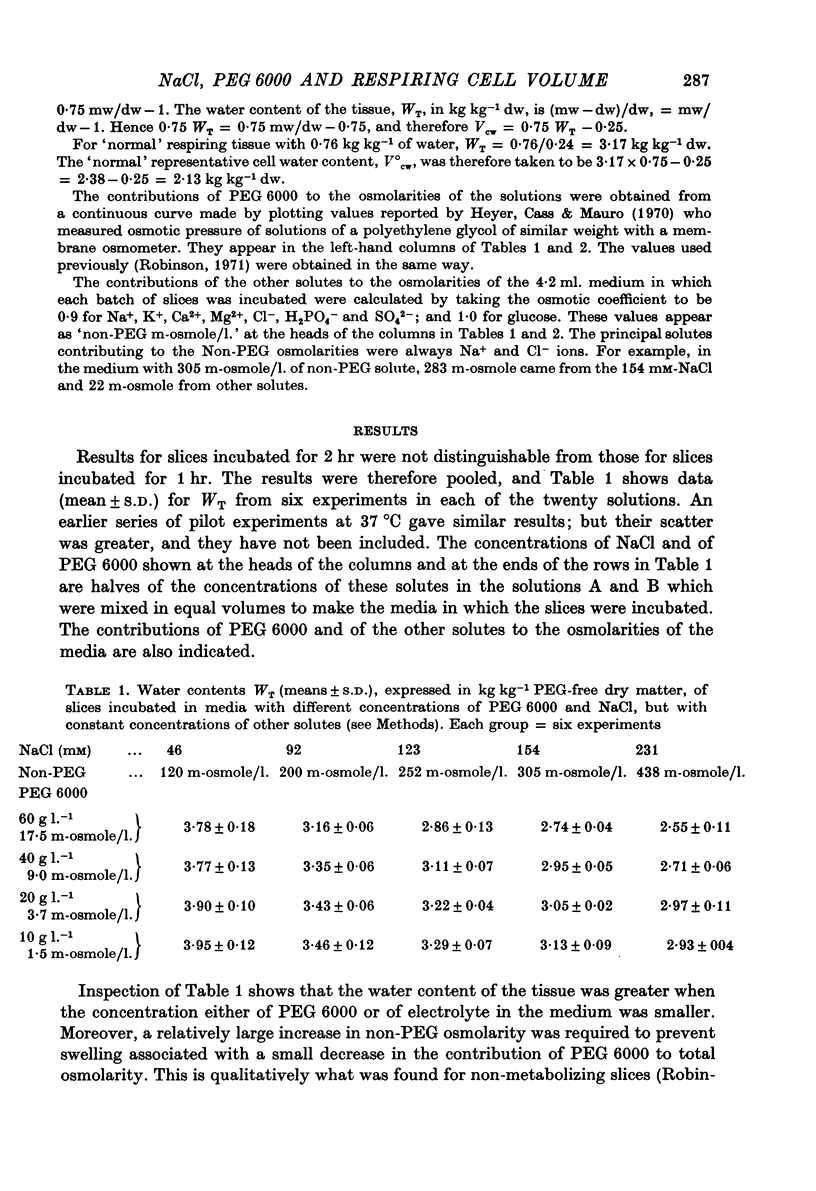
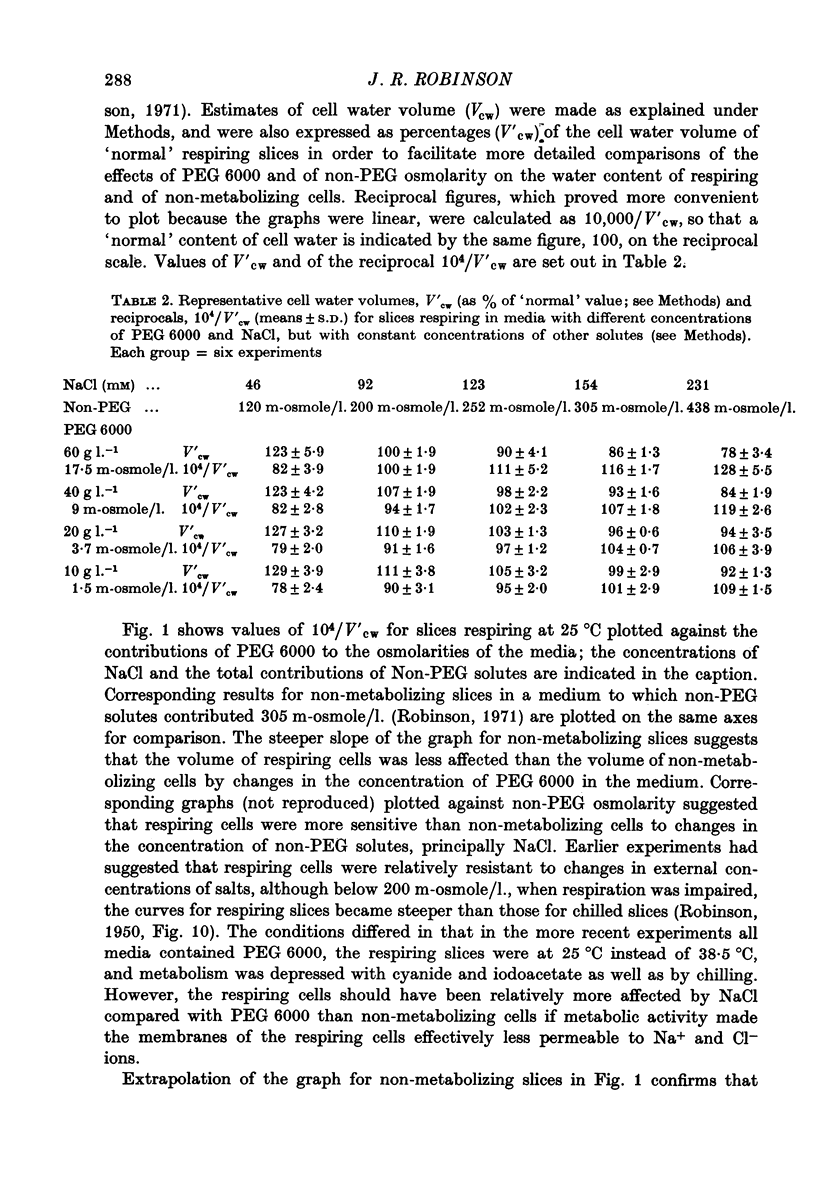
3. The slices contained more water when the concentration either of NaCl or of PEG 6000 was lower.
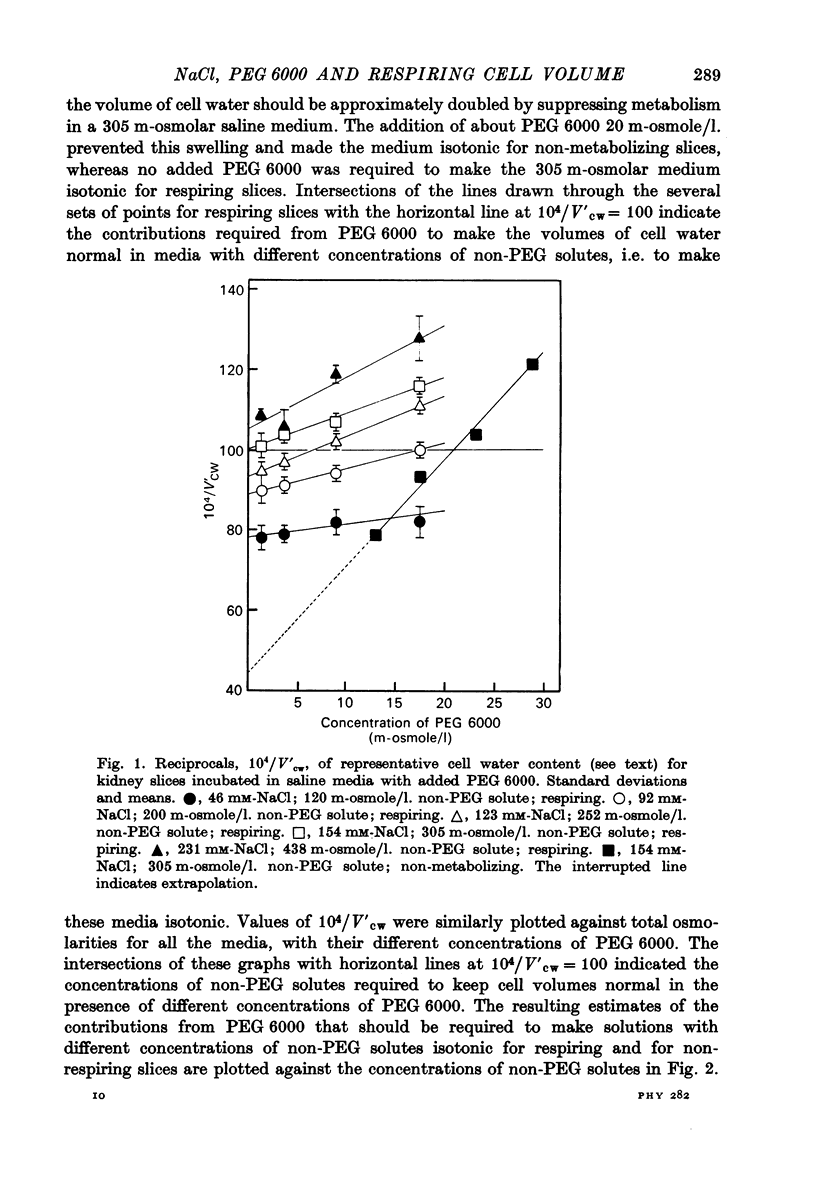
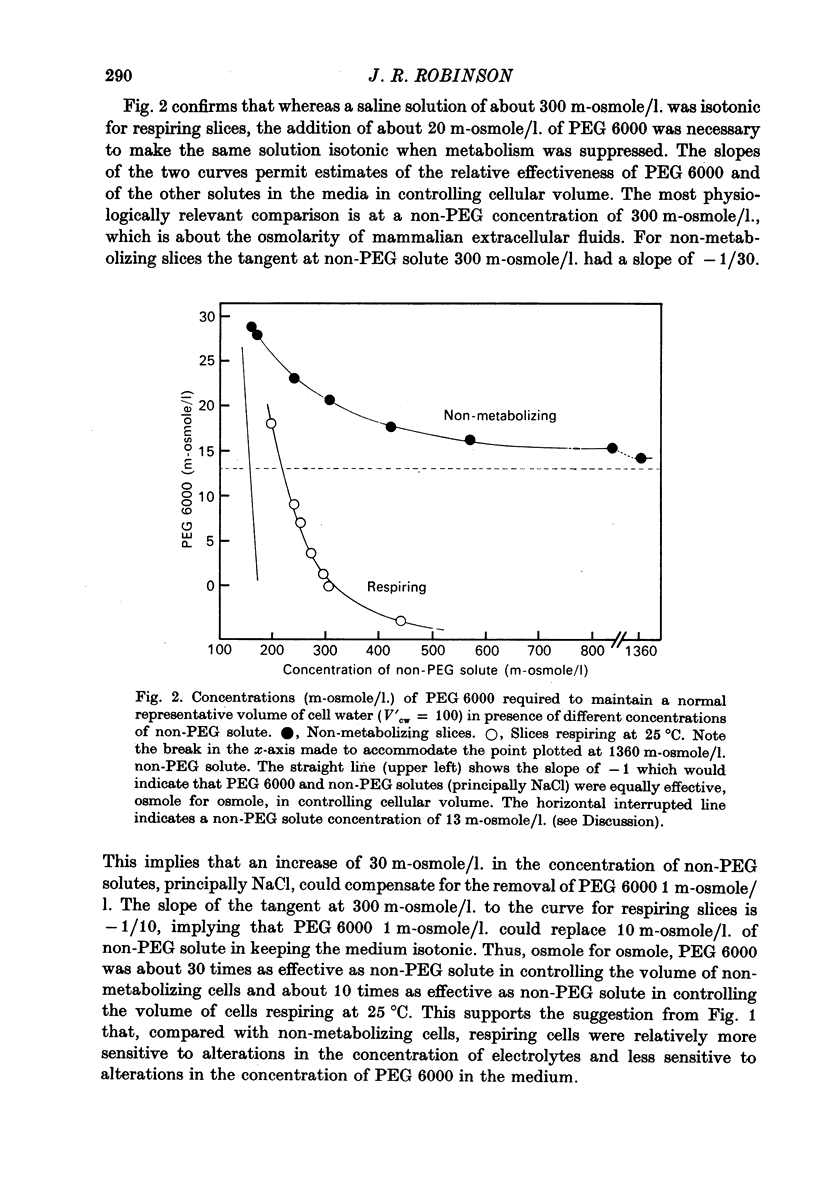
4. Larger increases in the contribution of NaCl to total osmolarity were required to prevent increases in water content accompanying relatively small reductions in the contribution of PEG 6000. At a physiological salt concentration around 300 m-osmole/l., it took 10 m-osmole/l. of additional NaCl to compensate for the loss of 1 m-osmole/l. PEG 6000 and keep the volume of cell water normal.
5. The much greater contribution, osmole for osmole, which PEG 6000 made to tonicity suggests that metabolic activity failed to make the cell membranes effectively semipermeable; they were still substantially permeable to NaCl.
6. Comparison with earlier results suggested that ionic strength affected colloid osmolarity similarly in respiring and in non-metabolizing cells; and that respiration at 25 °C somehow kept the concentration of diffusible solutes in the cells sufficiently lower than outside to compensate for a swelling effect of intracellular colloid osmolarity which, in the absence of metabolism, could be balanced by 20 m-osmole/l. PEG 6000 added to the medium.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Glaister D., Kerly M. The oxygen consumption and carbohydrate metabolism of the retractor muscle of the foot of Mytilus edulis. J Physiol. 1936 Jun 10;87(1):56–66. doi: 10.1113/jphysiol.1936.sp003388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer E., Cass A., Mauro A. A demonstration of the effect of permeant and impermeant solutes, and unstirred boundary layers on osmoti flow. Yale J Biol Med. 1969 Dec;42(3-4):139–153. [PMC free article] [PubMed] [Google Scholar]
- LEAF A. Maintenance of concentration gradients and regulation of cell volume. Ann N Y Acad Sci. 1959 Feb 6;72(12):396–404. doi: 10.1111/j.1749-6632.1959.tb44168.x. [DOI] [PubMed] [Google Scholar]
- McIver D. J., Macknight A. D. Extracellular space in some isolated tissues. J Physiol. 1974 May;239(1):31–49. doi: 10.1113/jphysiol.1974.sp010554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON J. R. Metabolism of intracellular water. Physiol Rev. 1960 Jan;40:112–149. doi: 10.1152/physrev.1960.40.1.112. [DOI] [PubMed] [Google Scholar]
- ROBINSON J. R. Osmoregulation in surviving slices from the kidneys of adult rats. Proc R Soc Lond B Biol Sci. 1950 Oct 13;137(888):378–402. doi: 10.1098/rspb.1950.0048. [DOI] [PubMed] [Google Scholar]
- ROBINSON J. R. The effect of sodium and chloride ions upon swelling of rat kidney slices treated with a mercurial diuretic. J Physiol. 1956 Oct 29;134(1):216–228. doi: 10.1113/jphysiol.1956.sp005638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. R. Control of water content of non-metabolizing kidney slices by sodium chloride and polyethylene glycol (PEG 6000). J Physiol. 1971 Feb;213(1):227–234. doi: 10.1113/jphysiol.1971.sp009378. [DOI] [PMC free article] [PubMed] [Google Scholar]


