Abstract
Brucella abortus is a facultative intracellular bacterium capable of surviving inside macrophages. Intracellular replication of B. abortus requires the VirB complex, which is highly similar to conjugative DNA transfer systems. In this study, we show that plasma membrane cholesterol of macrophages is required for the VirB-dependent internalization of B. abortus and also contributes to the establishment of bacterial infection in mice. The internalization of B. abortus was accelerated by treating macrophages with acetylated low-density lipoprotein (acLDL). Treatment of acyl coenzyme A:cholesterol acyltransferase inhibitor, HL-004, to macrophages preloaded with acLDL accelerated the internalization of B. abortus. Ketoconazole, which inhibits cholesterol transport from lysosomes to the cell surface, inhibited the internalization and intracellular replication of B. abortus in macrophages. The Niemann-Pick C1 gene (NPC1), the gene for Niemann-Pick type C disease, characterized by an accumulation of cholesterol in most tissues, promoted B. abortus infection. NPC1-deficient mice were resistant to the bacterial infection. Molecules associated with cholesterol-rich microdomains, “lipid rafts,” accumulate in intracellular vesicles of macrophages isolated from NPC1-deficient mice, and the macrophages yielded no intracellular replication of B. abortus. Thus, trafficking of cholesterol-associated microdomains controlled by NPC1 is critical for the establishment of B. abortus infection.
Brucella species are facultative intracellular pathogens that survive in a variety of cells, including macrophages, and their virulence and chronic infections are thought to be due to their ability to avoid the killing mechanisms within macrophages (1). The molecular mechanisms of their virulence and chronic infections are incompletely understood. Recent studies with HeLa cells have confirmed these observations, showing that Brucella inhibits phagosome-lysosome fusion and transits through an intracellular compartment that resembles autophagosomes. Bacteria replicate in a different compartment, containing protein markers normally associated with the endoplasmic reticulum, as shown by confocal microscopy and immunogold electron microscopy (5, 24). Brucella internalizes into macrophages by swimming on the cell surface, with generalized membrane ruffling for several minutes, after which the bacteria are enclosed by macropinosomes (33). “Lipid raft”-associated molecules, such as glycosylphosphatidylinositol (GPI)-anchored proteins, GM1 gangliosides, and cholesterol, have been selectively incorporated into macropinosomes containing Brucella abortus. The disruption of lipid rafts on macrophages markedly inhibits the VirB-dependent macropinocytosis and intracellular replication (33). These results indicated that replicative phagosome formation of B. abortus is modulated by lipid raft microdomains.
The operon coding for export mechanisms specializing in transferring a variety of multimolecular complexes across the bacterial membrane to the extracellular space or into other cells has been described previously (27). These complexes, named type IV secretion systems, are in B. abortus (virB genes) (27). This operon comprises 13 open reading frames that share a homology with other bacterial type IV secretion systems involved in the intracellular trafficking of pathogens. Type IV secretion systems export four types of substrates: (i) DNA conjugation intermediates; (ii) the multisubunit pertussis toxin; (iii) monomeric proteins, including primase, RecA, and the Agrobacterium tumefaciens VirE2 and VirF proteins; (iv) and the Helicobacter pylori CagA protein (4). The RalF protein has been identified as a substrate of the type IV secretion system of Legionella pneumophila (20). However, substrates of the VirB secretion system of B. abortus and the target of the secretion system in host cells is still unclear.
In this study, we investigated the roles of plasma membrane cholesterol in internalization by the VirB system and the establishment of B. abortus infection in mice. Plasma membrane cholesterol associates with lipid raft microdomains. Lipid raft microdomains were originally reported by Simons and van Meer to explain sphingolipid-based sorting properties in cellular membranes (28) and were later proposed to explain cholesterol-based microheterogeneities in the membrane. Plasma membrane cholesterol and intracellular cholesterol trafficking was therefore expected to contribute to internalization and intracellular replication of B. abortus in macrophages, because recent evidence indicates that cholesterol sequestration from macrophages inhibits the internalization and intracellular replication of B. abortus (21, 33). Our results show that the plasma membrane cholesterol not only influences the bacterial internalization and intracellular replication, but also contributes to the establishment of B. abortus infection.
MATERIALS AND METHODS
Bacterial strains and mice.
All B. abortus derivatives were from 544 (ATCC 23448) smooth virulent B. abortus biovar 1 strains. Ba598 (544 ΔvirB4) has been described previously (32). Plasmid pMAW114, which encodes green fluorescence protein (GFP), was constructed by cloning the BamHI-BglII fragment from the pQBI63 (GFP expression vector; TAKARA, Tokyo, Japan) into BamHI- and BglII-cleaved pBBR1MCS-2. pMAW114 (GFP+) was introduced into 544 (wild type) and Ba598 (ΔvirB4), and the derivatives were designated Ba600 (wild-type GFP+) and Ba604 (ΔvirB4 GFP+), respectively (33).
BALB/c mice carrying the genetic mutation for NPC1 were obtained from The Jackson Laboratory (Bar Harbor, Maine) (25).
Cell culture.
Bone marrow-derived macrophages from female BALB/c mice were prepared as described previously (32). The macrophages were seeded (2 × 105 to 3 × 105 in each well) in 24-well tissue culture plates for all assays. Macrophages were preloaded with or without acetylated low-density lipoprotein (acLDL) (50 μg/ml) and were treated with or without ketoconazole (10 mg/ml) or acyl coenzyme A:cholesterol acyltransferase (ACAT) inhibitor HL-004 (4 μg/ml; Taisho Pharmaceutical Co.) for 24 h (19).
Detection of intracellular bacteria by fluorescence microscopy.
B. abortus strains were grown to an A600 of 3.2 in brucella broth and were used to infect mouse bone marrow-derived macrophages for various periods at an indicated multiplicity of infection (MOI). Bacteria were deposited onto the macrophages by centrifugation at 150 × g for 5 min at room temperature. After 0-, 5-, 15-, 25-, and 35-min incubations at 37°C, infected macrophages were washed once with medium and were fixed in periodate-lysine-paraformaldehyde (16) containing 5% sucrose for 1 h at 37°C. The samples were washed three times in phosphate-buffered saline (PBS) and wells were successively incubated three times for 5 min in blocking buffer (2% goat serum in PBS) at room temperature. The samples were stained with anti-B. abortus polyclonal rabbit serum diluted 1:1,000 in blocking buffer to identify extracellular bacteria. After incubating for 1 h at 37°C, the samples were washed three times for 5 min with blocking buffer, were stained with Cascade blue-conjugated goat anti-rabbit immunoglobulin G diluted 1:500 in blocking buffer, and were incubated for 1 h at 37°C. The samples were washed three times and were mounted in mounting medium. One hundred macrophages were examined per coverslip to determine the total number of intracellular bacteria.
Determination of efficiency of intracellular growth of bacteria.
Bacteria were deposited onto macrophages at an MOI of 5 by centrifugation at 150 × g for 5 min at room temperature and then were incubated at 37°C in 5% CO2 for 1 h. Then the macrophages were washed once with RPMI medium and were incubated with 30 μg/ml gentamicin. At different time points, the cells were washed and lysed with distilled water, and the number of bacteria on plates of a suitable dilution was determined.
Virulence in mice.
The virulence was determined by quantitating the survival of the strains in the spleen after 10 days. Mice were injected intraperitoneally with approximately 104 CFU of brucellae in 0.1 ml of saline. Groups of five mice were injected with each strain. At 10 days after infection, their spleen was removed, weighed, and homogenized in saline. Tissue homogenates were serially diluted with PBS and were plated on Brucella agar to count the number of CFU in each spleen.
LAMP-1 staining.
Infected macrophages were fixed in periodate-lysine-paraformaldehyde-sucrose for 1 h at 37°C and stained for extracellular bacteria as described above. All antibody-probing steps were carried out for 1 h at 37°C. Samples were washed three times in PBS for 5 min and then permeabilized at −20°C in methanol for 10 s. After incubating three times for 5 min with blocking buffer, samples were stained with anti-LAMP-1 rat monoclonal antibody 1D4B diluted 1:100 in blocking buffer (30). After washing three times for 5 min in blocking buffer, samples were stained simultaneously with Texas red-conjugated goat anti-rat immunoglobulin G. Samples were placed in mounting medium and visualized by fluorescence microscopy. Intracellular bacteria were detected by GFP fluorescence and absence of staining with Cascade blue.
Fluorescence labeling of lipid raft-associated molecules.
Detection of the localization of GM1 gangliosides with cholera toxin B subunit (CTB) (10 μg/ml), GPI-anchored protein with aerolysin (2.5 μg/ml), and cholesterol with filipin (50 mg/ml) was described previously (33).
RESULTS
Acceleration of bacterial internalization by preloading cholesterol into macrophages.
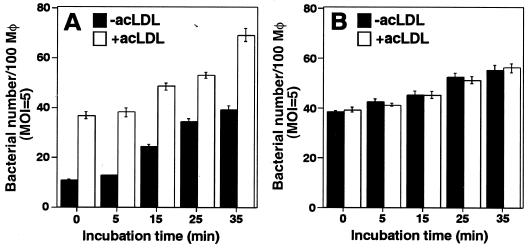
A prominent biological property of acLDL is its ability to induce lipid loading of macrophages in culture, which has been a useful model of the formation of lipid-laden macrophages (Fig. 1) (18, 19, 25). To investigate if intracellular cholesterol affects B. abortus internalization into macrophages, bacteria were deposited onto macrophages that were preloaded with acLDL, and intracellular bacteria were quantitated microscopically at various times of incubation. The bacteria were deposited onto macrophages by centrifugation, and then Ba604 (ΔvirB4) was rapidly internalized, with most of the associated bacteria internalized before further incubation at 37°C. In contrast, internalization of Ba600 (wild-type) was delayed and attained the same levels of internalization as Ba604 (ΔvirB4) only after 25 min of incubation (33). Internalization of Ba600 (wild-type) accelerated by preloading with acLDL into macrophages, but internalization of Ba604 (ΔvirB4) was not affected (Fig. 2). The percentage of bacteria in macropinosomes did not differ between macrophages treated with and without acLDL (data not shown).
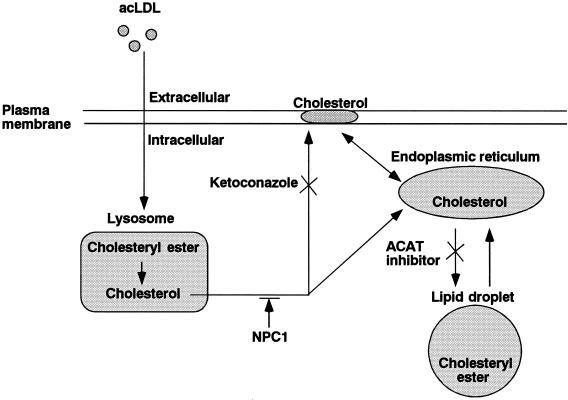
FIG. 1.
Schema of cholesterol trafficking in macrophages. The effect of each pharmacological and genetic treatment examined in this study is shown.
FIG. 2.
Internalization of B. abortus accelerated by acLDL. Ba600 (wild type) (A) or Ba604 (ΔvirB4) (B) was deposited onto bone marrow-derived macrophages with (white bars) or without (black bars) acLDL treatment and incubated at 37°C for the periods of time indicated. One hundred macrophages were examined per coverslip. Data are the average of triplicate samples from three identical experiments, and the error bars represent the standard deviation.
Effects of agents that modulate cholesterol trafficking on bacterial internalization and intracellular replication.
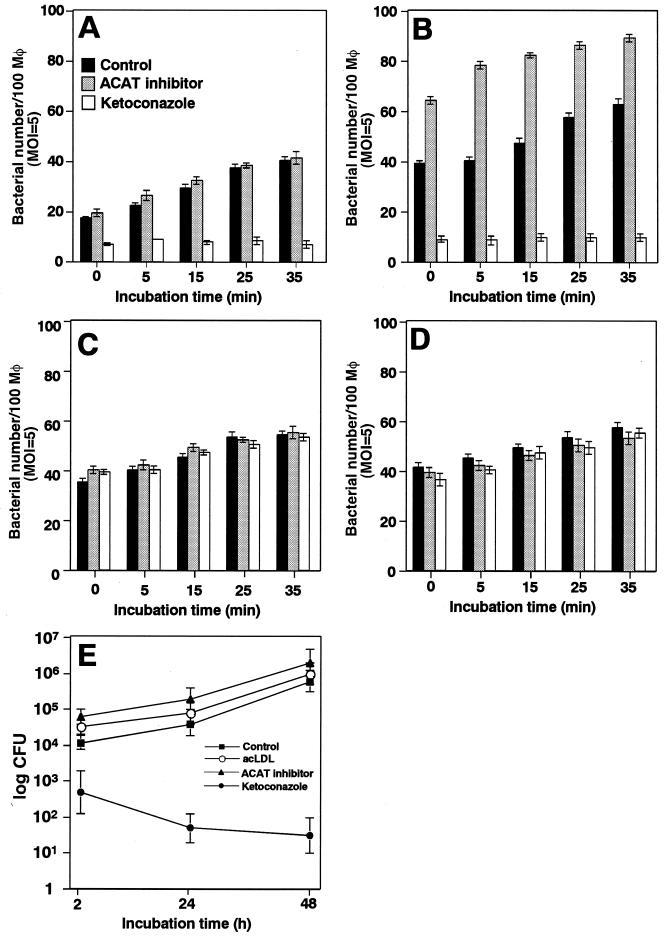
Macrophages take up modified lipoproteins by receptor-mediated endocytosis, and then the lipoproteins are delivered to lysosomes for degradation. This cholesterol is believed to mix with the bulk of cholesterol in the plasma membrane. Excess plasma membrane cholesterol then enters the cytoplasm, where the cholesterol is reesterified by ACAT and is stored in cellular lipid droplets (Fig. 1) (19). To investigate if intracellular cholesterol trafficking affects B. abortus internalization into macrophages, bacteria were deposited onto macrophages that were pretreated with ACAT inhibitor HL-004, and intracellular bacteria were quantitated microscopically at various times of incubation. HL-004 treatment accelerated the internalization of Ba600 (wild-type) into macrophages preloaded with acLDL (Fig. 3B). Under the same conditions, HL-004 did not accelerate the internalization of Ba604 (ΔvirB4) (Fig. 3C and D). Macropinosome formation of Ba600 (wild-type) was also enhanced by HL-004 treatment, but the percentage of bacteria in macropinosomes did not differ (data not shown). These results suggest that intracellular cholesterol transport would contribute to VirB-dependent internalization and macropinocytosis of B. abortus.
FIG. 3.
Internalization of B. abortus into macrophages was influenced by plasma membrane cholesterol. Ba600 (wild-type) (A and B) or Ba604 (ΔvirB4) (C and D) was deposited onto bone marrow-derived macrophages with ketoconazole (white bars) or with ACAT inhibitor HL-004 (gray bars), or without drug treatment (black bars) in the presence (B and D) or absence (A and C) of acLDL and incubated at 37°C for the periods of time indicated. One hundred macrophages were examined per coverslip. Data are the average of triplicate samples from three identical experiments, and the error bars represent the standard deviation. (E) Ketoconazole inhibited intracellular replication of B. abortus in macrophages. Macrophages in the presence or absence of ketoconazole, ACAT inhibitor HL-004, or acLDL were infected with Ba600 (wild type) as described in Materials and Methods. Data points and error bars represent the mean CFU of triplicate samples from a typical experiment (performed at least four times) and their standard deviation, respectively.
To confirm that plasma membrane cholesterol contributes to B. abortus internalization, we tested the effect of ketoconazole, which inhibits cholesterol transport from lysosomes to the cell surface (Fig. 1). Ketoconazole greatly diminished the internalization of Ba600 (wild-type) into macrophages preloaded with or without acLDL (Fig. 3A and B), but under the same conditions, it did not block the internalization of Ba604 (ΔvirB4) (Fig. 3C and D).
To determine whether intracellular cholesterol trafficking has a role in bacterial replication in macrophages, macrophages were treated with acLDL, ketoconazole, or HL-004 and then were infected with Ba600 (wild type). As reported previously (32, 33), Ba600 (wild-type) replicated in macrophages without ketoconazole treatment, but Ba600 (wild-type) failed to replicate in macrophages treated with ketoconazole (Fig. 3E). Although 12% ± 2.0% of internalized Ba600 (wild type) was observed under ketoconazole treatment (mean ± standard deviation) (Fig. 3A and B), the internalized bacteria did not replicate in the macrophages. Intracellular replication was not affected by acLDL and HL-004 (Fig. 3E). We consistently found that approximately 15% of internalized Ba600 (wild-type) into untreated macrophages target improperly into a LAMP-1 positive compartment (32, 33). These results suggest that other uptake pathways of B. abortus by macrophages exist, but replicative phagosome formation requires the uptake pathway associated with plasma membrane cholesterol.
Role of cholesterol trafficking in establishment of B. abortus infection.
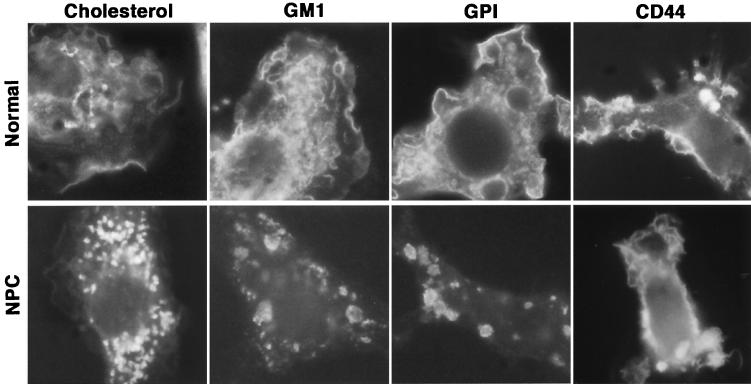
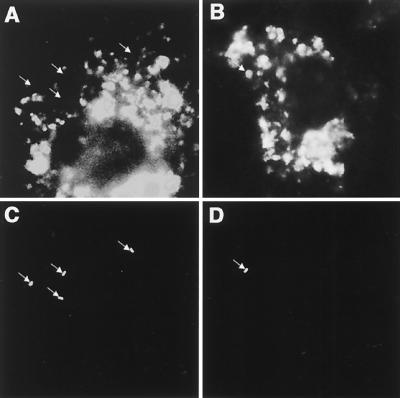
The most prominent cellular feature of Niemann-Pick type C (NPC) disease is lysosomal accumulation of free cholesterol, caused by impaired relocation of cholesterol derived from LDL from the lysosome to other cellular sites, such as the plasma membrane and endoplasmic reticulum (Fig. 1) (23). To investigate if NPC1 contributes to the recruitment of lipid raft-associated molecules, fluorescence-labeled lipid raft-associated molecules, such as cholesterol, GM1 gangliosides, and GPI-anchored proteins, were observed by microscopy. These molecules were in the plasma membrane and intracellular vesicles of macrophages from wild-type mice (Fig. 4). In contrast, these molecules accumulated only in intracellular vesicles in macrophages from NPC1-deficient mice (Fig. 4). Localization of the transmembrane protein CD44, which is not associated with lipid rafts, was not affected by NPC1 (Fig. 4). These results suggest that NPC1 influences lipid raft formation on the macrophage surface.
FIG. 4.
Intracellular distribution of lipid raft-associated molecules in NPC1-deficient macrophages. Macrophages of wild-type (upper panels) or NPC1-deficient (lower panels) mice were labeled with indicated molecules.
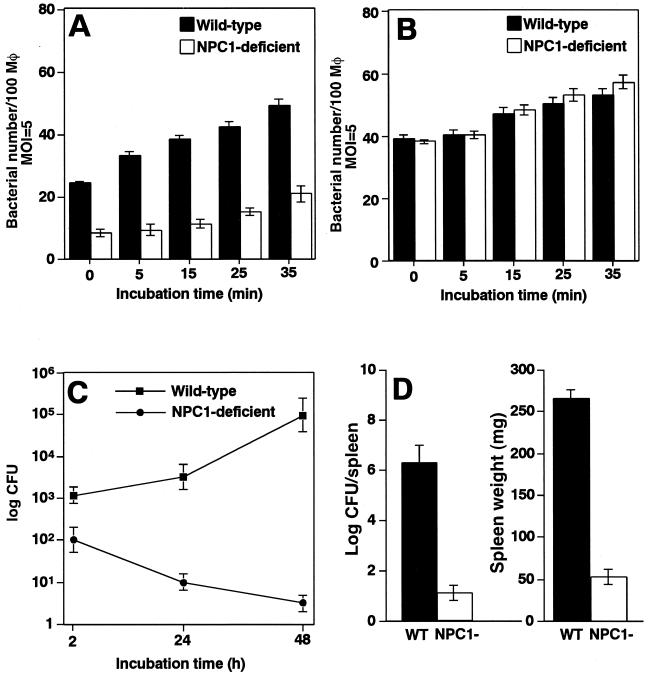
We next investigated if NPC1 contributes to the internalization and intracellular replication of B. abortus. B. abortus was infected to bone marrow derived-macrophages from wild-type or NPC1-deficient BALB/c mice, and the intracellular bacteria were quantitated microscopically at various periods of incubation. Macrophages from wild-type mice supported internalization and intracellular replication of Ba600 (wild-type), but not macrophages from NPC1-deficient mice (Fig. 5A). Fewer bacteria were internalized in macrophages from NPC1-deficient mice, but they did not replicate in the macrophages (Fig. 5C). Macrophages from wild-type and NPC1-deficient mice showed no significant difference in the internalization of Ba604 (ΔvirB4) (Fig. 5B). In NPC1-deficient mice, Ba600 (wild-type) failed to block phagosome maturation, as shown by colocalization of phagosomes containing the bacteria and the late endocytic marker, LAMP-1, at 1 h after infection (82.8% ± 3.4% positive) (Fig. 6B and D). In contrast, Ba600 (wild-type) prevented phagosome-lysosome fusion, and therefore phagosomes containing Ba600 (wild-type) do not have endocytic and lysosomal marker proteins in macrophages from wild-type mice (Fig. 6A and C). These results suggest that replicative phagosome formation required uptake pathway associated with NPC1.
FIG. 5.
NPC1-influenced B. abortus infection. (A and B) Internalization of B. abortus. Macrophages from wild-type (black bars) or NPC1-deficient (white bars) mice were infected with virulent Ba600 (wild type) (A) or Ba604 (ΔvirB4) (B) for the periods of time indicated. Data are the average of triplicate samples from three identical experiments, and the error bars represent the standard deviation. (C) Intracellular replication of B. abortus. Macrophages from wild-type or NPC1-deficient mice were infected with Ba600 (wild type). Data points and error bars represent the mean CFU of triplicate samples from a typical experiment (performed at least four times) and their standard deviation, respectively. (D) Proliferation in mice. Wild-type (black bar) or NPC1-deficient (white bars) mice were infected with virulent B. abortus. Recovery of viable bacteria from the spleen and the weights of spleens of infected mice at 10 days postinfection are shown. Error bars indicate standard deviations.
FIG. 6.
Colocalization of B. abortus with late endosomal and lysosomal marker LAMP-1 in macrophages from NPC1-deficient mice by immunofluorescence microscopy. Macrophages from wild-type mice (A and C) or NPC1-deficient mice (B and D) were infected with Ba600 (wild type) for 1 h, fixed, and stained for LAMP-1 colocalization (A and B) and intracellular bacteria (C and D). Arrows point to bacteria.
To find if this defect in internalization and intracellular replication of B. abortus correlates with an inability to establish infection in the host, we experimentally infected wild-type or NPC1-deficient mice with B. abortus. Many bacteria were recovered from the spleen of wild-type mice infected with Ba600 (wild type) at 10 days after infection, but fewer bacteria were recovered from NPC1-deficient mice, based on the number of CFU in each spleen (Fig. 5D). These results indicated that B. abortus proliferation was promoted by NPC1.
DISCUSSION
In this study we showed that internalization and intracellular replication of B. abortus in mouse macrophages are influenced by plasma membrane cholesterol and that intracellular cholesterol trafficking is essential to establish B. abortus infection in the mouse model. These events were dependent on the presence of the VirB system. Studies of the cellular trafficking of cholesterol derived from the metabolism of LDL showed that after hydrolysis of LDL cholesteryl ester in lysosomes, most LDL-derived cholesterol traffics to the plasma membrane (14). The internalization of B. abortus into macrophages was accelerated by preloading with acLDL, indicating that the cholesterol on the plasma membrane of macrophages contributes to the internalization of B. abortus. To confirm this possibility, we used two inhibitors of intracellular cholesterol trafficking, HL-004 and ketoconazole. As HL-004 is a specific ACAT inhibitor (18), alterations in cholesterol metabolism by HL-004 can be attributed to the intracellular ACAT inhibition in macrophages (19). Macrophages incorporate modified LDL via scavenger receptors, which is not down-regulated by cellular sterol levels (8). The incorporated cholesteryl ester is delivered to lysosomes, and is hydrolyzed to free cholesterol, which forms the intracellular free cholesterol pool. Excess free cholesterol is esterified by ACAT and the cholesteryl ester is stored in cytoplasmic inclusions. This cholesteryl ester is the substrate for neutral cholesteryl ester hydrolase. Thus, a cholesteryl ester cycle of de-esterification exists because of the hydrolase and reesterification by ACAT. Free cholesterol can move between intracellular pools and the plasma membrane (3). Internalization and intracellular replication of B. abortus in macrophages were modulated by cholesterol-rich microdomains, so-called lipid rafts, on plasma membrane surfaces (33). As the cholesterol microdomains are induced in macrophages when esterification of excess LDL-derived cholesterol is blocked with ACAT inhibitor (10), the internalization of B. abortus by uptake pathway associated with lipid rafts into macrophages increased by ACAT inhibitor treatment. In contrast, ketoconazole treatment greatly diminished the internalization of B. abortus into macrophages. Ketoconazole interferes with trafficking of cholesterol from lysosomes to the plasma membrane (12). As the appearance of cholesterol microdomains can be inhibited by ketoconazole (10), the internalization of B. abortus by uptake pathway associated with lipid rafts into macrophages decreases upon ketoconazole treatment. These results suggest that the plasma membrane cholesterol should influence the internalization of B. abortus. Fewer B. abortus cells were internalized into macrophages treated with ketoconazole, but the internalized bacteria did not replicate, suggesting that replicative phagosome formation would require correct intracellular cholesterol trafficking and plasma membrane cholesterol.
To confirm this hypothesis, macrophages from NPC1-deficient mice were infected with B. abortus. The gene that causes NPC disease, referred to as NPC1, has been mapped to a region of chromosome 18 in both humans and mice and has been cloned (15). Although the function of NPC1 remains undefined, studies have shown a crucial role for this protein in cholesterol metabolism (13). NPC1-deficient mice share many of the pathophysiological abnormalities observed in patients with NPC, including accumulation of cholesterol in tissues (15). Our results showed that lipid raft-associated molecules accumulate in intracellular vesicles in macrophages from NPC1-deficient mice. NPC1 is recruited to the site of free cholesterol accumulation by enrichment of cellular cholesterol or by pharmacological intervention of cholesterol egress from the lysosomes (34). Intracellular trafficking of GM1 ganglioside in NPC1-deficient Chinese hamster ovary cells has been shown by using CTB as a probe (29). CTB-labeled vesicles contain the early endosome marker Rab5 but not LAMP-2, indicating that they represent early endosomes. Similarly, CTB accumulate in intracellular vesicles of human NPC fibroblasts that contain both Rab5 and early endosomal antigen 1 (29). Presumably, these results, together with our results, indicate that cholesterol or GM1 ganglioside accumulate in lysosomes or early endosomes in macrophages from NPC1-deficient mice. Therefore, the internalization of B. abortus by uptake pathway associated with lipid rafts was inhibited in macrophages from NPC1-deficient mice.
The role of mouse macrophages in mediating resistance or susceptibility among mouse strains to some intracellular pathogens has been shown by studies of the Ity/Lsh/Bcg resistance model; resistance to Salmonella enterica serovar Typhimurium, Leishmania donovani, and mycobacterial species is regulated by the polymorphism of the Nramp1 gene that controls macrophage function (6). Bovine Nramp1 is a major candidate for controlling the in vivo resistant phenotype against B. abortus infection (2). Our results indicate that NPC1 promotes the internalization and intracellular replication of B. abortus and also contributes to bacterial proliferation in mice. However, control of B. abortus infections is a multigenic trait (9), and further investigation is needed to clarify the genetic control of B. abortus infection.
Cholesterol or GPI-anchored proteins is included in apicomplexan Toxoplasma gondii and Plasmodium falciparum vacuoles (11, 17). Cholesterol is essential for the uptake of Mycobacterium bovis by macrophages (7). Cholesterol accumulates at the site of M. bovis entry, depleting plasma membrane cholesterol specifically inhibits M. bovis uptake, and M. bovis has a high binding capacity for cholesterol (7). Macropinosomes harboring L. pneumophila also include lipid raft-associated macromolecules (31). A similar process of selective lipid recruitment has been described during human immunodeficiency virus or influenza virus budding from mammalian cells (22, 26). Since lipid rafts are thought to be involved in signaling pathways in immune cells, uptake processes associated with lipid rafts might lead microorganisms into compartments that avoid fusion with the lysosomal network, and that is essential for the establishment of infection. The results of this study will provide a new target of prevention against infection by intracellular pathogens.
Acknowledgments
This work was supported, in part, by grants-in-aid for scientific research (12575029 and 13770129), Japan Society for the Promotion of Science, and by the Sasakawa Scientific Research Grant from The Japan Science Society.
Editor: D. L. Burns
REFERENCES
- 1.Baldwin, C. L., and A. J. Winter. 1994. Macrophages and Brucella. Immunol. Ser. 60:363-380. [PubMed] [Google Scholar]
- 2.Barthel, R., J. Feng, J. A. Piedrahita, D. N. McMurray, J. W. Templeton, and L. G. Adams. 2001. Stable transfection of the bovine NRAMP1 gene into murine RAW264.7 cells: effect on Brucella abortus survival. Infect. Immun. 69:3110-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, M. S., Y. K. Ho, and J. L. Goldstein. 1980. The cholesteryl ester cycle in macrophage foam cells: continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J. Biol. Chem. 255:9344-9352. [PubMed] [Google Scholar]
- 4.Christie, P. J., and J. P. Vogel. 2000. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comerci, D. J., M. J. Martinez-Lorenzo, R. Sieira, J. Gorvel, and R. A. Ugalde. 2001. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 3:159-168. [DOI] [PubMed] [Google Scholar]
- 6.Forbes, J. R., and P. Gros. 2001. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 9:397-403. [DOI] [PubMed] [Google Scholar]
- 7.Gatfield, J., and J. Pieters. 2000. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288:1647-1650. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein, J. L., Y. K. Ho, S. K. Basu, and M. S. Brown. 1979. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA 76:333-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho, M., and C. Cheers. 1982. Resistance and susceptibility of mice to bacterial infection. IV. Genetic and cellular basis of resistance to chronic infection with Brucella abortus. J. Infect. Dis. 146:381-387. [DOI] [PubMed] [Google Scholar]
- 10.Kruth, H. S., I. Ifrim, J. Chang, L. Addadi, D. Perl-Treves, and W.-Y. Zhang.2001. Monoclonal antibody detection of plasma membrane cholesterol microdomains responsive to cholesterol trafficking. J. Lipid Res. 42:1492-1500. [PubMed] [Google Scholar]
- 11.Lauer, S., J. VanWye, T. Harrison, H. McManus, B. U. Samuel, N. L. Hiller, N. Mohandas, and K. Haldar. 2000. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 19:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liscum, L. 1990. Pharmacological inhibition of the intracellular transport of low-density lipoprotein-derived cholesterol in Chinese hamster ovary cells. Biochim. Biophys. Acta 1045:40-48. [DOI] [PubMed] [Google Scholar]
- 13.Liscum, L., and J. J. Klansek. 1998. Niemann-Pick disease type C. Curr. Opin. Lipidol. 9:131-135. [DOI] [PubMed] [Google Scholar]
- 14.Liscum, L., and N. J. Nunn. 1999. Intracellular cholesterol transport. Biochim. Biophys. Acta 1438:19-37. [DOI] [PubMed] [Google Scholar]
- 15.Loftus, S. K., J. A. Morris, E. D. Carstea, J. Z. Gu, C. Cummings, A. Brown, J. Ellison, K. Ohno, M. A. Rosenfeld, D. A. Tagle, P. G. Pentchev, and W. J. Pavan. 1997. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science 277:232-235. [DOI] [PubMed] [Google Scholar]
- 16.McLean, I. W., and P. K. Nakane. 1974. Periodate-lysine-paraformaldehyde fixative: a new fixative for immunoelectron microscopy. J. Histochem. Cytochem. 22:1077-1083. [DOI] [PubMed] [Google Scholar]
- 17.Mordue, D. G., N. Desai, M. Dustin, and L. D. Sibley. 1999. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J. Exp. Med. 190:1783-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami, S., H. Araki, S. Otomo, Y. Nara, and Y. Yamori. 1995. Effects of HL-004, a novel ACAT inhibitor, on cholesterol accumulation and removal in cultured smooth muscle cell from stroke-prone spontaneously hypertensive rats (SHRSP). Life Sci. 56:509-520. [DOI] [PubMed] [Google Scholar]
- 19.Murakami, S., I. Yamagishi, Y. Asami, M. Sato, and K. Tomisawa. 1996. Effect of the ACAT inhibitor, HL-004, on cholesterol metabolism in macrophages. Cell. Mol. Biol. 42:865-872. [PubMed] [Google Scholar]
- 20.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295:679-682. [DOI] [PubMed] [Google Scholar]
- 21.Naroeni, A., and F. Porte. 2002. Role of cholesterol and the ganglioside GM1 in entry and short-term survival of Brucella suis in murine macrophages. Infect. Immun. 70:1640-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono, A., and E. O. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 98:13925-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pentchev, P. G., M. T. Vanier, K. Suzuki, and M. C. Patterson. 1995. XVI. Lysosomal disorders, p. 2625-2640. In C. R. Seriver, A. L. Beaudet, W. S. Sly, and D. Valle (ed.), The metabolic and molecular basis of inherited disease. McGraw-Hill Inc., New York, N.Y.
- 24.Pizarro-Cerda, J., E. Moreno, V. Sanguedolce, J. L. Mege, and J. P. Gorvel. 1998. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect. Immun. 66:2387-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawamura, N., J.-S. Gong, W. S. Garver, R. A. Heidenreich, H. Ninomiya, K. Ohno, K. Yanagisawa, and M. Michikawa. 2001. Site-specific phosphorylation of Tau accompanied by activation of mitogen-activated protein kinase (MAPK) in brains of Niemann-Pick type C mice. J. Biol. Chem. 276:10314-10319. [DOI] [PubMed] [Google Scholar]
- 26.Scheiffele, P., A. Rietveld, T. Wilk, and K. Simons. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 27.Sieira, R., D. J. Comerci, D. O. Sanchez, and R. A. Ugalde. 2000. A homologue of an operon required for DNA transfer in Agrobacterium tumefaciens is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 182:4849-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simons, K., and G. van Meer. 1988. Lipid sorting in epithelial cells. Biochemistry. 27:6197-6202. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto, Y. H. Ninomiya, Y. Ohsaki, K. Higaki, J. P. Davies, Y. A. Ioannou, and K. Ohno. 2001. Accumulation of cholera toxin and GM1 ganglioside in the early endosome of Niemann-Pick C1-deficient cells. Proc. Natl. Acad. Sci. USA 98:12391-12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swanson, M. S., and R. R. Isberg. 1996. Identification of Legionella pneumophila mutants that have aberrant intracellular fates. Infect. Immun. 64:2585-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watarai, M., I. Derre, J. Kirby, J. D. Growney, W. F. Dietrich, and R. R. Isberg. 2001. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J. Exp. Med. 194:1081-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watarai, M., S.-I. Makino, and T. Shirahata. 2002. An essential virulence protein of Brucella abortus, VirB4, requires an intact nucleoside triphosphate-binding domain. Microbiology 148:1439-1446. [DOI] [PubMed] [Google Scholar]
- 33.Watarai, M., S.-I. Makino, Y. Fujii, K. Okamoto, and T. Shirahata. 2002. Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell. Microbiol. 4:341-355. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, M., N. K. Dwyer, E. B. Neufeld, D. C. Love, A. Cooney, M. Comly, S. Patel, H. Watari, J. F. Strauss III, P. G. Pentchev, J. A. Hanover, and E. J. Blanchette-Mackie. 2001. Sterol-modulated glycolipid sorting occurs in Niemann-Pick C1 late endosomes. J. Biol. Chem. 276:3417-3425. [DOI] [PubMed] [Google Scholar]