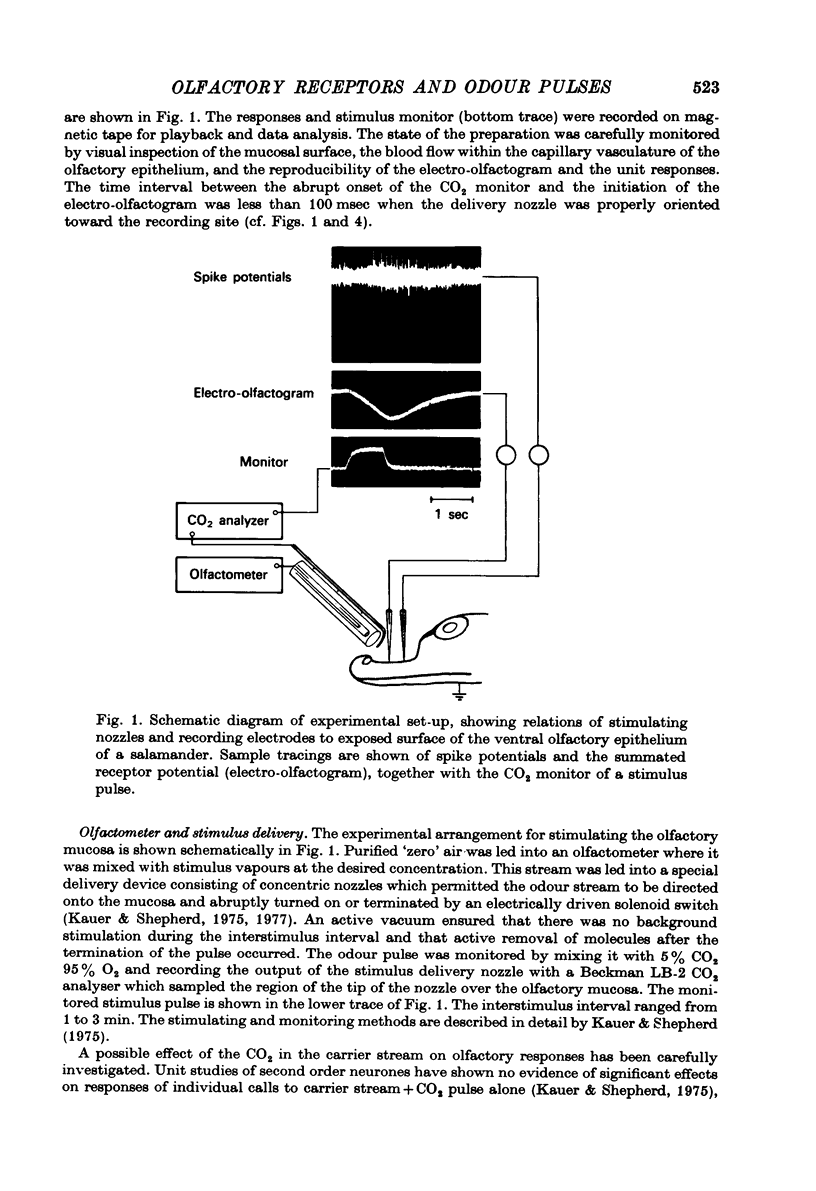
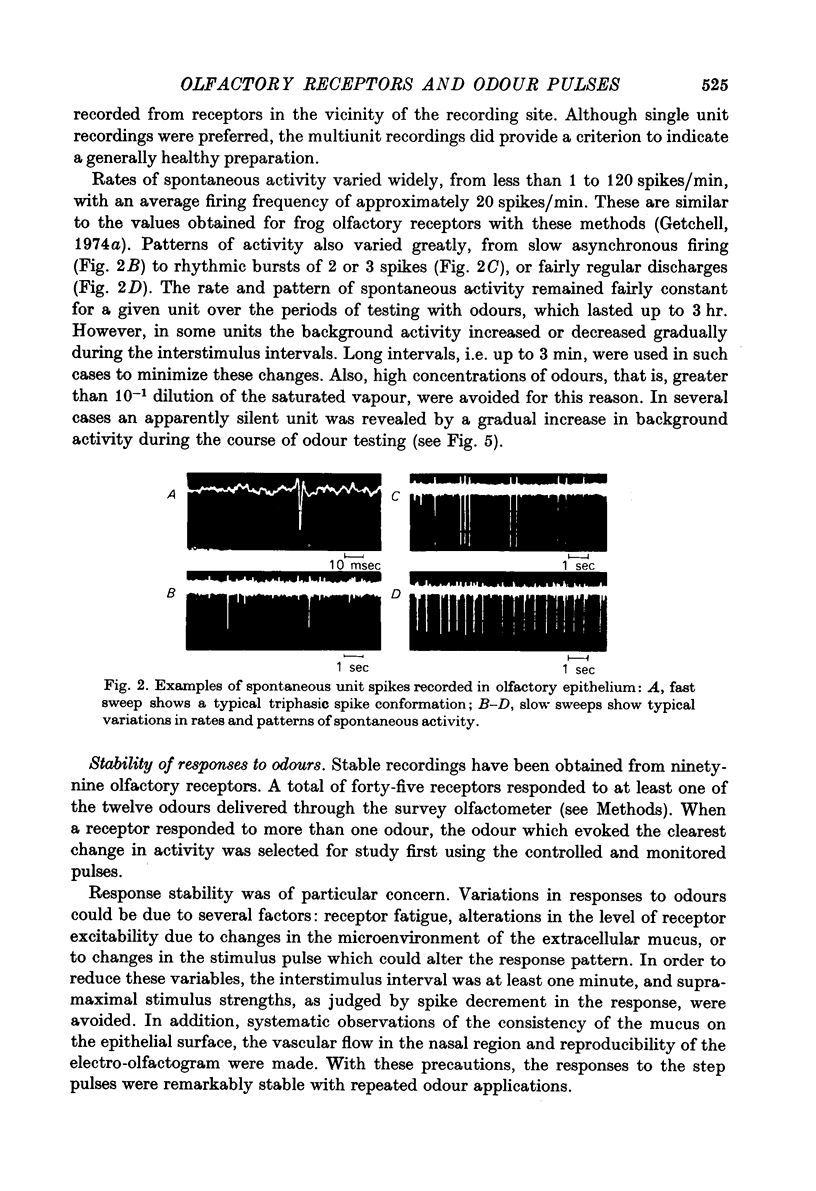
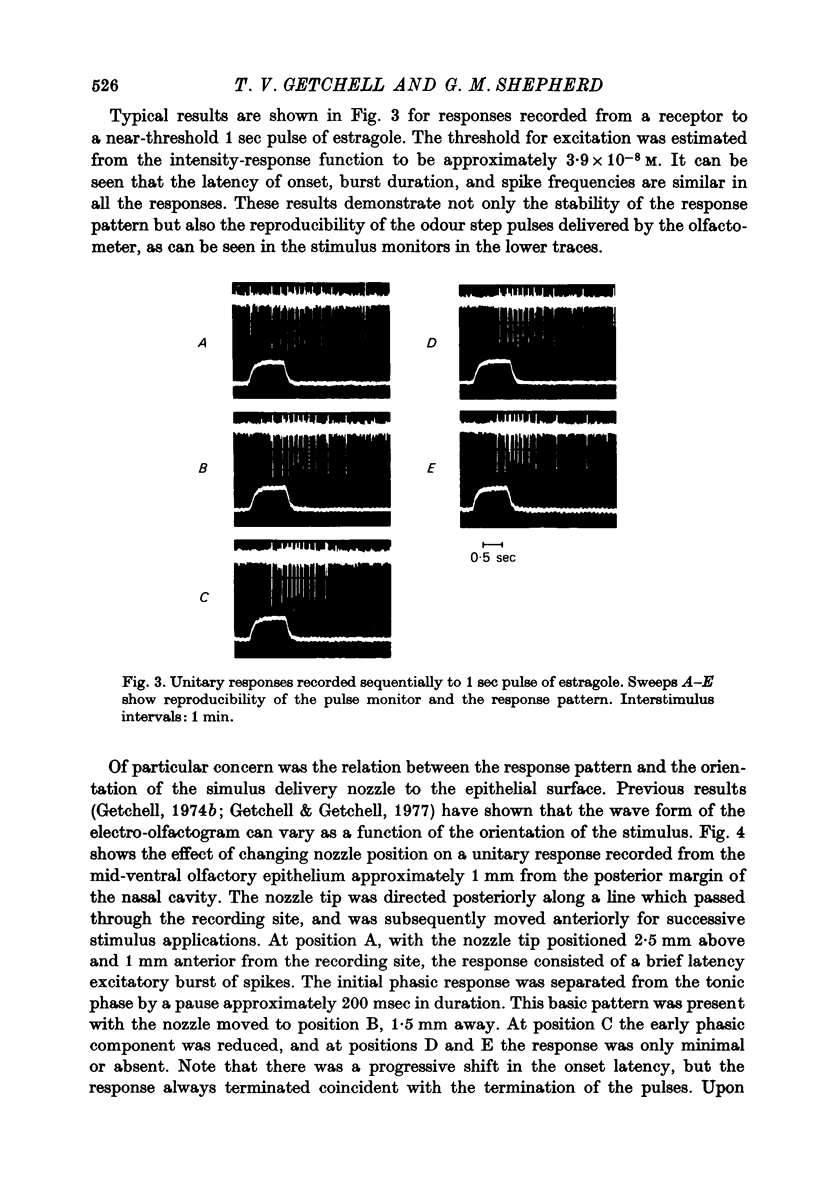
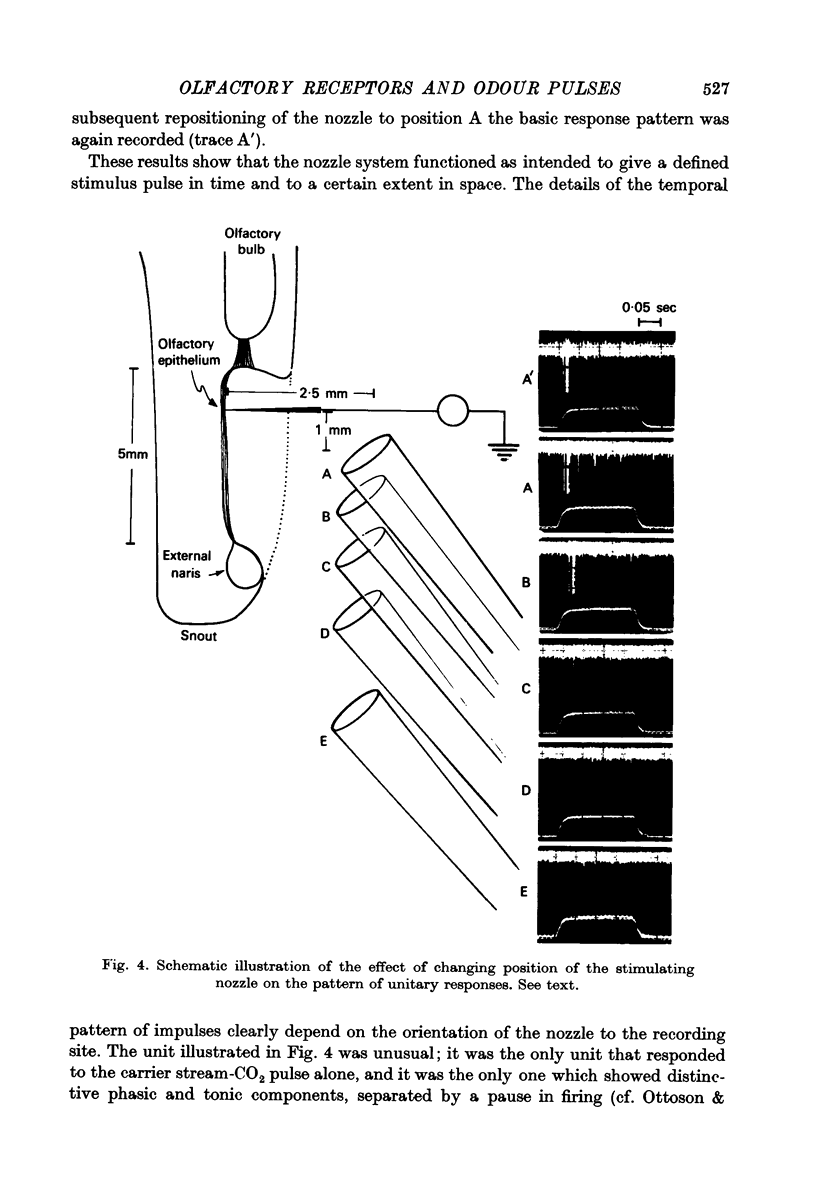
Abstract
1. The response properties of single olfactory receptor cells in the salamander have been analysed in unitary recordings obtained with platinum-black metal-filled micro-electrodes. 2. Stimulation has been carried out using an apparatus which delivers odour pulses of abrupt onset, steady plateau and abrupt termination. The pulses have been monitored near the site of stimulation on the olfactory epithelium during the experiments. 3. The main type of response was a discharge of impulses that was time locked to the stimulus pulse. The pattern of the responses consisted of a relatively brief latency of onset, a rapid rise in impulse frequency, a continuation of impulse firing during the plateau of the pulse, and an abrupt termination of the discharge correlated with the termination of the pulse. 4. There was a clear relationship between the receptor responses and odour concentration. In general, impulse firing frequency increased with increasing odour concentration. The firing frequency ranged from approximately 1--3 impulses/sec at threshold, up to 20 impulses/sec at the highest concentration. 5. Two types of reduced impulse activity were observed. One occurred after the termination of the pulse and lasted 1--3 sec; this was a common occurrence. The other type was seen during a pulse as a reduction of impulse activity compared to the background level; this type was rarely observed. 6. The receptor responses resembled those of mitral cells in the olfactory bulb to odour pulses in their sensitivity to odour concentration. They differed in that mitral cells show primary response categories consisting of brief excitation followed by suppression, and pure suppression, that are rarely seen at the receptor level. These differences may be ascribed to synaptic interactions in the olfactory bulb. 7. It is concluded that the majority of receptor cells have a stereotyped discharge response pattern and a systematic relation to odour concentration. These properties appear to reflect the simple time course of the odour pulses used in these experiments. This represents an initial step toward analysing olfactory coding at the receptor level using stimuli controlled in a manner similar to that used in other sensory systems.
Full text
PDF


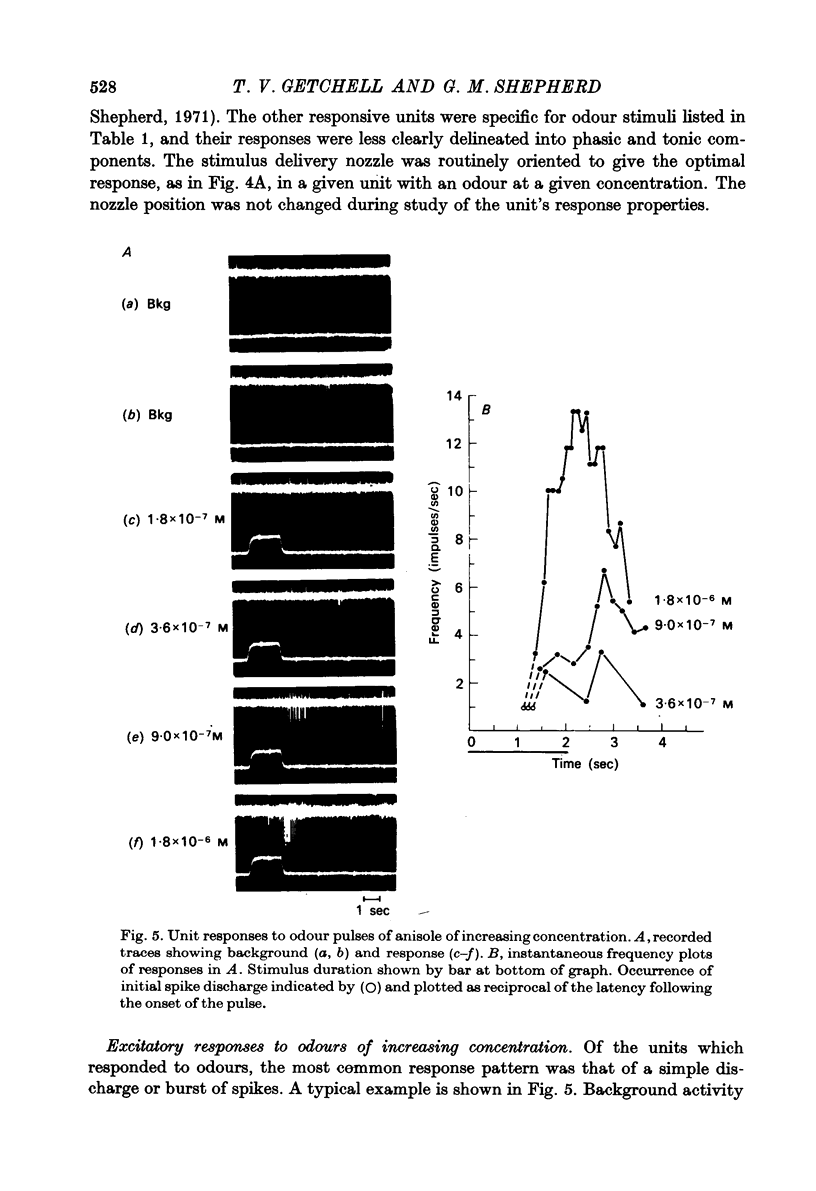

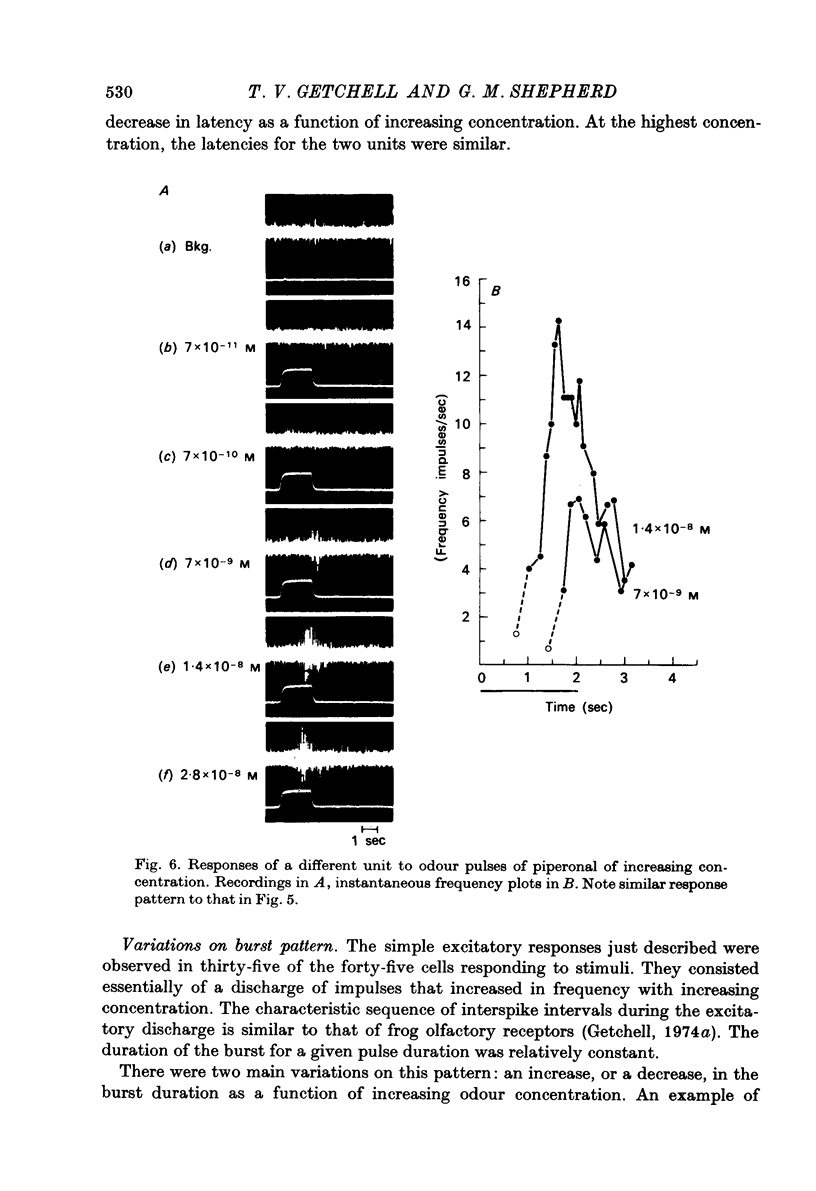
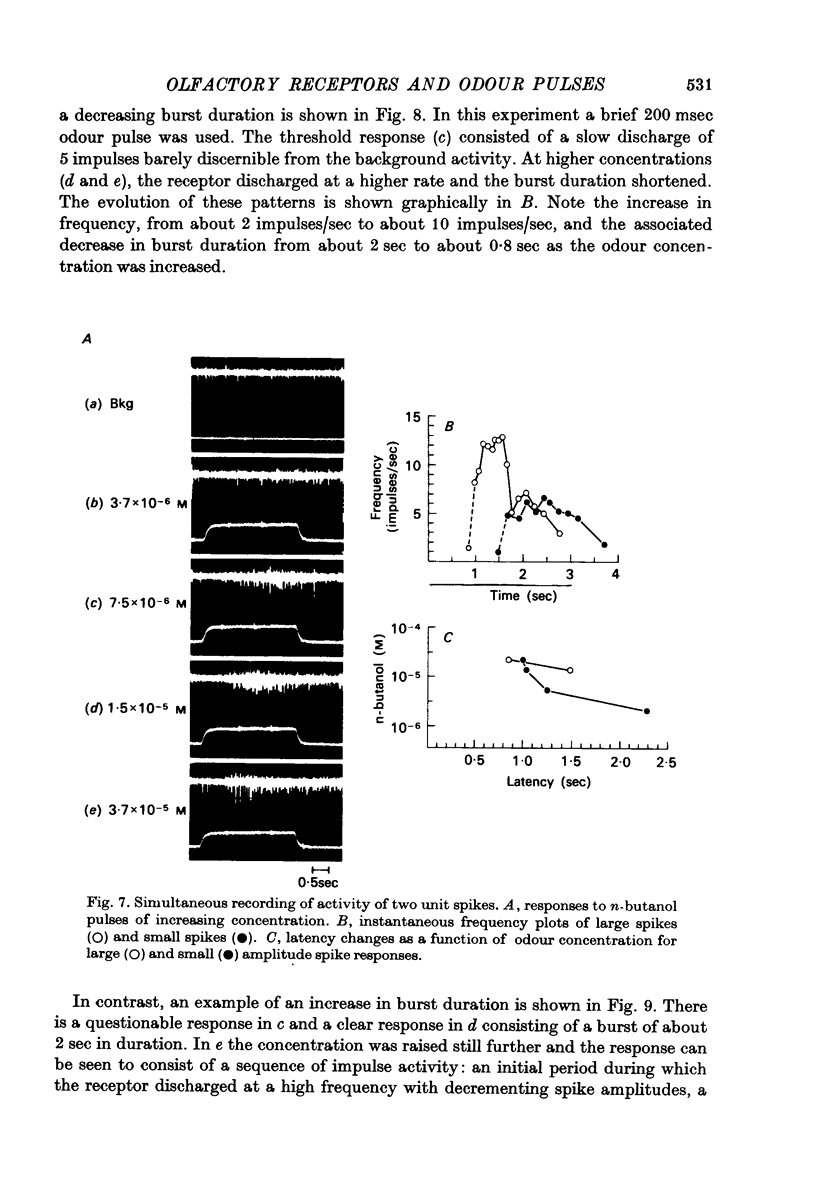
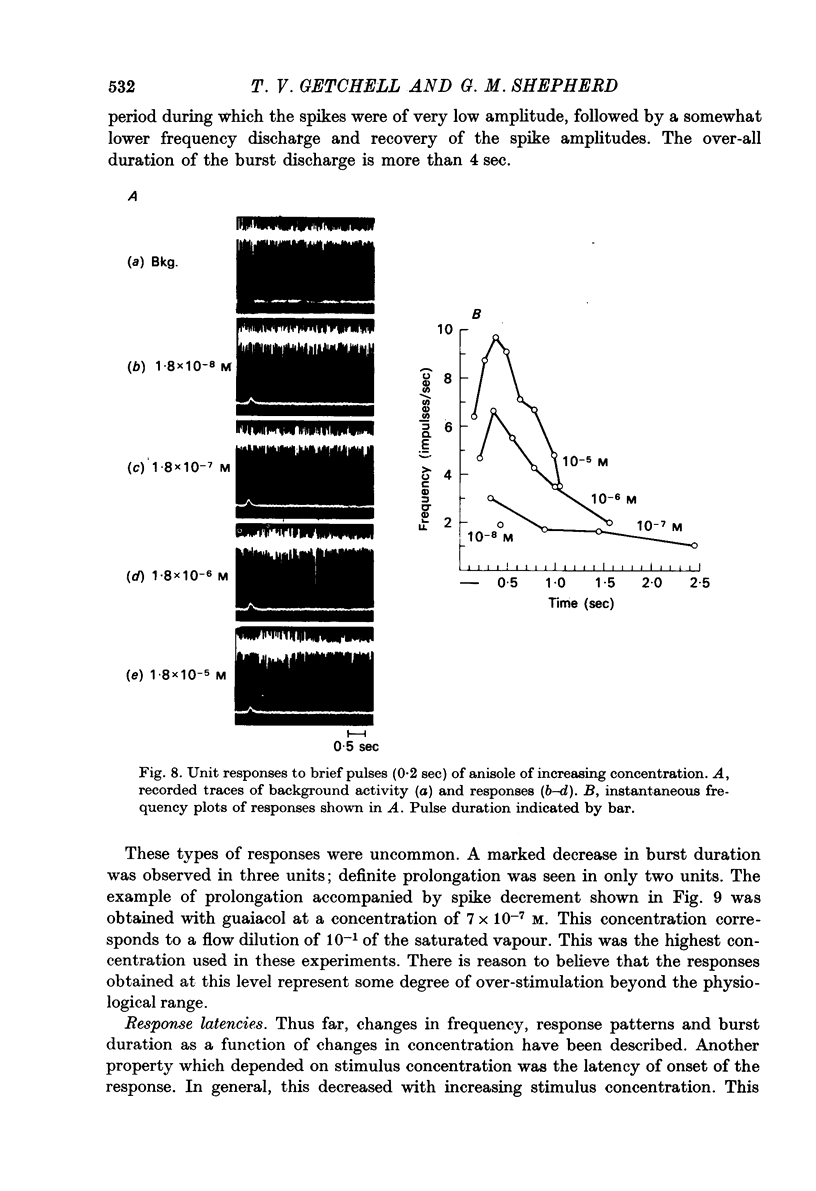





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blank D. L. Mechanism underlying the analysis of odorant quality at the level of the olfactory mucosa. II. Receptor selective sensitivity. Ann N Y Acad Sci. 1974 Sep 27;237(0):91–101. doi: 10.1111/j.1749-6632.1974.tb49846.x. [DOI] [PubMed] [Google Scholar]
- Gesteland R. C., Lettvin J. Y., Pitts W. H. Chemical transmission in the nose of the frog. J Physiol. 1965 Dec;181(3):525–559. doi: 10.1113/jphysiol.1965.sp007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V. Analysis of intracellular recordings from salamander olfactory epithelium. Brain Res. 1977 Mar 11;123(2):275–286. doi: 10.1016/0006-8993(77)90479-6. [DOI] [PubMed] [Google Scholar]
- Getchell T. V. Analysis of unitary spikes recorded extracellularly from frog olfactory receptor cells and axons. J Physiol. 1973 Nov;234(3):533–551. doi: 10.1113/jphysiol.1973.sp010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V. Electrogenic sources of slow voltage transients recorded from frog olfactory epithelium. J Neurophysiol. 1974 Nov;37(6):1115–1130. doi: 10.1152/jn.1974.37.6.1115. [DOI] [PubMed] [Google Scholar]
- Getchell T. V., Getchell M. L. Signal-detecting mechanisms in the olfactory epithelium: molecular discrimination. Ann N Y Acad Sci. 1974 Sep 27;237(0):62–75. doi: 10.1111/j.1749-6632.1974.tb49844.x. [DOI] [PubMed] [Google Scholar]
- Getchell T. V., Shepherd G. M. Adaptive properties of olfactory receptors analysed with odour pulses of varying durations. J Physiol. 1978 Sep;282:541–560. doi: 10.1113/jphysiol.1978.sp012480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V., Shepherd G. M. Short-axon cells in the olfactory bulb: dendrodendritic synaptic interactions. J Physiol. 1975 Oct;251(2):523–548. doi: 10.1113/jphysiol.1975.sp011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V., Shepherd G. M. Synaptic actions on mitral and tufted cells elicited by olfactory nerve volleys in the rabbit. J Physiol. 1975 Oct;251(2):497–522. doi: 10.1113/jphysiol.1975.sp011105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V. Unitary responses in frog olfactory epithelium to sterically related molecules at low concentrations. J Gen Physiol. 1974 Aug;64(2):241–261. [PMC free article] [PubMed] [Google Scholar]
- Graziadei P. P., Monti Graziadei G. A. Olfactory epithelium of Necturus maculosus and Ambystoma tigrinum. J Neurocytol. 1976 Feb;5(1):11–32. doi: 10.1007/BF01176180. [DOI] [PubMed] [Google Scholar]
- Holley A., Duchamp A., Revial M. F., Juge A. Qualitative and quantitative discrimination in the frog olfactory receptors: analysis from electrophysiological data. Ann N Y Acad Sci. 1974 Sep 27;237(0):102–114. doi: 10.1111/j.1749-6632.1974.tb49847.x. [DOI] [PubMed] [Google Scholar]
- Hornung D. E., Mozell M. M. Factors influencing the differential sorption of odorant molecules across the olfactory mucosa. J Gen Physiol. 1977 Mar;69(3):343–61. doi: 10.1085/jgp.69.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer J. S., Moulton D. G. Responses of olfactory bulb neurones to odour stimulation of small nasal areas in the salamander. J Physiol. 1974 Dec;243(3):717–737. doi: 10.1113/jphysiol.1974.sp010773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer J. S., Shepherd G. M. Analysis of the onset phase of olfactory bulb unit responses to odour pulses in the salamander. J Physiol. 1977 Nov;272(2):495–516. doi: 10.1113/jphysiol.1977.sp012056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer J. S., Shepherd G. M. Olfactory stimulation with controlled and monitored step pulses of odor. Brain Res. 1975 Feb 21;85(1):108–113. doi: 10.1016/0006-8993(75)91014-8. [DOI] [PubMed] [Google Scholar]
- Mathews D. F. Response patterns of single neurons in the tortoise olfactory epithelium and olfactory bulb. J Gen Physiol. 1972 Aug;60(2):166–180. doi: 10.1085/jgp.60.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell R. J., Mozell M. M. Quantitative stimulation of frog olfactory receptors. J Neurophysiol. 1969 Jan;32(1):51–63. doi: 10.1152/jn.1969.32.1.51. [DOI] [PubMed] [Google Scholar]
- OTTOSON D. Analysis of the electrical activity of the olfactory epithelium. Acta Physiol Scand Suppl. 1955;35(122):1–83. [PubMed] [Google Scholar]
- SHIBUYA T., SHIBUYA S. Olfactory epithelium: unitary responses in the tortoise. Science. 1963 May 3;140(3566):495–496. doi: 10.1126/science.140.3566.495. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Tucker D. Amino acids as olfactory stimuli in freshwater catfish, Ictalurus catus (Linn.). Comp Biochem Physiol A Comp Physiol. 1971 Oct;40(2):399–404. doi: 10.1016/0300-9629(71)90030-2. [DOI] [PubMed] [Google Scholar]


