Abstract
We tested the immunogenicity of two Trypanosoma cruzi antigens injected into mice in the form of DNA vaccine. Immunization with DNA encoding dihydroorotate dehydrogenase did not confer protective immunity in all mouse strains tested. Immunization with DNA encoding trans-sialidase surface antigen (TSSA) protected C57BL/6 (H-2b) mice but not BALB/c (H-2d) or C3H/Hej (H-2k) mice against lethal T. cruzi infection. In vivo depletion of CD4+ or CD8+ T cells abolished the protective immunity elicited by TSSA gene in C57BL/6 mice. Enzyme-linked immunospot assay with splenocytes from T. cruzi-infected mice or TSSA gene-vaccinated mice identified an H-2Kb-restricted antigenic peptide, ANYNFTLV. The CD8+-T-cell line specific for this peptide could recognize T. cruzi-infected cells in vitro and could protect naive mice from lethal infection when adoptively transferred. Coadministration of the interleukin-12 (IL-12) gene with the TSSA gene facilitated the induction of ANYNFTLV-specific CD8+ T cells and improved the vaccine efficacy against lethal T. cruzi infection. These results reinforced the utility of immunomodulatory adjuvants such as IL-12 gene for eliciting protective immunity against intracellular parasites by DNA vaccination.
Trypanosoma cruzi is an etiological agent of Chagas' disease (7, 17, 18, 31). As it replicates inside cells after infecting mammalian hosts, cellular immune responses are critical for resolving its infection. In accordance with this notion, several studies have demonstrated that CD8+ T cells are particularly important for controlling the acute phase of its infection (34, 37, 42, 43, 44). Recent identification of CD8+-T-cell epitopes of T. cruzi antigens reinforced the importance of this effector cell population in protective immunity (9, 20, 36, 47, 48).
Vaccines that can elicit CD8+-T-cell responses have been eagerly expected for combating intracellular pathogens and several provocative strategies have been reported, including recombinant viruses carrying target antigens (21, 29, 41), linear peptides (16), lipopeptides (12, 24, 26), and DNA vaccines (1, 14). The prime-boost vaccination strategy was revolutionary since it could strongly enhance the CD8+-T-cell-dependent protective immunity (27, 33, 50). The same strategy was also effective against T. cruzi infection, as demonstrated by using a well-characterized model antigen for antigen-specific CD8+-T-cell induction (25). However, more efficient vaccination regimens must be explored for future human applications.
In order to develop better vaccination regimens, we first identified a T. cruzi antigen that could induce CD8+-T-cell responses by DNA vaccination. To achieve this, we first randomly chose two recently cloned and characterized parasite antigens from gene cluster encoding enzymes for de novo pyrimidine biosynthesis and others (10) to test their immunogenicity. Dihydroorotate dehydrogenase (DHOD) is one of the six enzymes responsible for de novo pyrimidine biosynthesis, whereas trans-sialidase surface antigen (TSSA) belongs to T. cruzi trans-sialidase superfamily members (39) but lacks the C-terminal repeats. Then, we tested coadministration of an immunomodulatory interleukin-12 (IL-12) gene to determine whether it could enhance the T. cruzi antigen-specific CD8+-T-cell response. IL-12 is a strong adjuvant for inducing T-cell-mediated immunity (11, 13, 35, 46). Administration of recombinant IL-12 could improve the prognosis of T. cruzi infection (15) and IL-12 gene transfer was also effective for controlling the infection (38, 49). However, a comprehensive study using the IL-12 gene as an adjuvant has not been performed against experimental Chagas' disease. In this study, we examined the vaccine efficacy of DNA encoding a specific T. cruzi antigen in combination with DNA encoding IL-12 and demonstrated that this combination could greatly improve the protective immunity against this protozoan disease.
MATERIALS AND METHODS
Animals and parasite.
Female C57BL/6 (H-2b), BALB/c (H-2d), and C3H/HeJ (H-2k) mice, 5 to 8 weeks of age, were purchased from Japan SLC (Hamamatsu, Shizuoka, Japan). Blood-form trypomastigotes of T. cruzi Tulahuen strain (22) were maintained in outbred CD1 or inbred BALB/c mice by the intramuscular (i.m.) inoculation of 5,000 of them into naive mice every 2 weeks.
Cells and culture.
The C57BL/6-derived thymoma cell line EL-4 was used as antigen-presenting cells for CD8+-T-cell cultures and assays. The Swiss 3T3 fibroblast cell line was used for producing T. cruzi trypomastigotes for in vitro infection. These cells were cultured in high-glucose Dulbecco modified Eagle medium (DMEM; Life Technologies/BRL, Rockville, Md.) supplemented with 10% fetal calf serum, 2 g of sodium bicarbonate (Sigma, St. Louis, Mo.)/liter, 200 mg of l-arginine hydrochloride (Life technoloGies/BRL)/liter, 36 mg of l-asparagine (Life Technologies/BRL)/liter, 2.6 g of HEPES (Sigma)/liter, 5 × 10−5 M 2-mercaptoethanol (Sigma), and antibiotics (complete DMEM). The medium used for the enzyme-linked immunospot (ELISPOT) assay, and the culture of lymphocytes was supplemented with phorbol myristate acetate-stimulated EL-4 cell culture supernatant as a source of 30 U of IL-2/ml (complete DMEM-IL-2). Insect-form (epimastigotes) of T. cruzi Tulahuen strain was cultured in LIT medium (4), incubated at 27°C, and then cocultured with Swiss 3T3 cells to produce tissue culture trypomastigotes as previously described (25).
T. cruzi infection in vitro.
EL-4 cells (4 × 106) were cocultured with 2 × 107 T. cruzi trypomastigotes in 15 ml of complete DMEM for 30 h (the host cell/parasite ratio was 1:5) (25). This infection condition was the same as that in a Swiss 3T3 fibroblast cell line and usually resulted in infection of 100% cells, as estimated by microscopic observation.
Construction of plasmid DNA.
pCMV-Tag mammalian expression vector (pCMV; Stratagene, La Jolla, Calif.) was used for expressing T. cruzi antigen. DHOD cDNA and TSSA cDNA, which had been cloned from T. cruzi Tulahuen strain previously (10), were supplied by T. Nara (Juntendo University School of Medicine), incorporated into the pCMV vector at the BamHI site, and designated pDHOD and pTSSA, respectively. Murine IL-12 cDNA, which contains IL-12 p40, the internal ribosome entry site of equine encephalomyocarditis virus, and IL-12 p35, was a kind gift from U. Gubler (Hoffmann-La Roche) and M. Toda (Keio University School of Medicine) (45). It was recloned into pcDNA3.1(−) (Invitrogen, Carlsbad, Calif.) at EcoRV and AflII sites and was designated pcIL-12.
Peptides.
The following six peptides that contained the potential H-2Kb- and H-2Db-binding motif (32) were selected from the amino acid sequence of TSSA (10) and synthesized: TC1 (ANYNFTLV; residues 536 to 543), TC2 (QLYHFANYNFTLV; residues 531 to 543), TC3 (GFNPNKAPI; residues 684 to 692), TC4 (SEDANNNKI; residues 173 to 181), TC5 (SRHLFYSAML; residues 2 to 11), and TC6 (QCKKNGESDIFTGV; residues 77 to 84). Their purity and integrity were confirmed by high-pressure liquid chromatography (HPLC) analysis. Peptide VDYNFTIV, which has been reported to be a CD8+-T-cell epitope (47), was also synthesized and verified by HPLC.
Establishment of peptide-specific CD8+-T-cell line and adoptive transfer.
Splenocytes (5 × 107) from C57BL/6 mice that had been infected with nonlethal dose of T. cruzi 4 months before were weekly stimulated with X-irradiated EL-4 cells (3 × 106) that were pulsed with 1 μM peptide and X-irradiated naive C57BL/6 splenocytes (2 × 107) as a feeder in complete DMEM-IL-2. Antigenic specificity of the cultured T cells was monitored weekly by ELISPOT assay. When the peptide-specific T cells exceeded 50% of the total cells, they were incubated with anti-B220 monoclonal antibody (MAb; RA3-3A1, ATCC) and anti-CD4 MAb (GK1.5; American Type Culture Collection), together with complement for depleting B and CD4+ T cells. Flow cytometric analysis confirmed that more than 95% of the peptide-specific T cells were CD8+. The possible contamination of small number of viable, infective T. cruzi in culture was excluded by following reasons. The culture of a T-cell line that was stimulated by antigenic peptide weekly for more than 1 year has never exhibited signs of pathological changes. Considering the parasite's potent capacity for proliferation in vitro coresiding with host cells, the lack of pathological changes for more than 1 year has strongly suggested that there is no contamination of infective T. cruzi in culture. In addition, the T cells died quickly without weekly antigenic peptide stimulation, indicating that there is no trace of parasite antigen in culture. To ensure this, we performed PCR aiming for the amplification of TSSA fragment from the lysate of 105 T-cell line. The reaction detected the presence of as few as 10 T. cruzi epimastigotes, while it detected no specific 2.1-kb band in 105 T cells (data not shown).
One week after the last stimulation, 5 × 107 cells were adoptively transferred into naive C57BL/6 mice via tail vein three times at 1-week intervals. As a control, the same number of splenocytes from naive C57BL/6 mice were transferred. These mice were infected with 1,000 blood-form T. cruzi trypomastigotes intraperitoneally (i.p.) at the time of first cell transfer.
DNA immunization and challenge.
Each mouse was i.m. injected with 100 μg of plasmid DNA dissolved in 50 μl of sterile phosphate-buffered saline (PBS) into the right hind leg quadriceps two to four times at 10-day intervals. These mice were i.m. inoculated with 5,000 or 10,000 T. cruzi blood-form trypomastigotes, 10 to 14 days after the last immunization. We found that i.m. infection causes the most virulent course of T. cruzi infection, as reflected by both parasitemia and percent survival. An i.p. infection causes the intermediate and an intravenous infection results in the attenuation of infection for most of the infected mice. By changing the dose and the route of infection, we found that we could detect the subtle differences of immunological effect in vivo among several experimental groups. For this reason, we chose several combinations of infection dose and route of infection. All infected mice were bled from tail vein periodically, and the number of parasites in 5 μl of blood was determined microscopically. The survival of all mice was monitored daily.
Quantification of antigen-specific T cells by ELISPOT assay.
Frequency of antigen-specific T cells was determined by ELISPOT assay for gamma interferon (IFN-γ)-secreting cells essentially as described previously (5, 23). Briefly, serial dilutions of splenocytes or T cells (1 × 104 to 100 × 104) were cocultured with irradiated EL-4 cells that had been pulsed with 1 μM peptide in anti-IFN-γ MAb-coated plate for 24 to 28 h. The spots formed by IFN-γ-secreting cells were detected with biotinylated anti-IFN-γ MAb, followed by peroxidase-labeled streptavidin and diaminobenzidine. The developed spots were counted under microscope and expressed as the number of spots per 106 cells.
In vivo depletion of CD8+ or CD4+ T cells.
C57BL/6 mice were i.p. injected with 1 mg of anti-CD8 (2.4.3; American Type Culture Collection) or anti-CD4 (GK1.5) MAb for three consecutive days until the day of challenge infection. As a control, normal rat immunoglobulin G (IgG; Sigma) was injected in the same protocol. Depletion of respective cells in the spleen was verified by flow cytometry.
Statistical analysis.
Statistical analyses were performed by the unpaired Student's t test for the ELISPOT assays and for the count of parasitemia. The unpaired Mann-Whitney U-test determined significant differences in survival data. P values of <0.05 were considered significant.
RESULTS AND DISCUSSION
pDHOD was ineffective for conferring protective immunity in all mouse strains tested, whereas pTSSA was effective only for C57BL/6 mice.
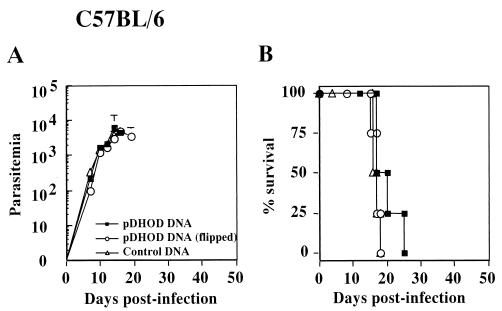
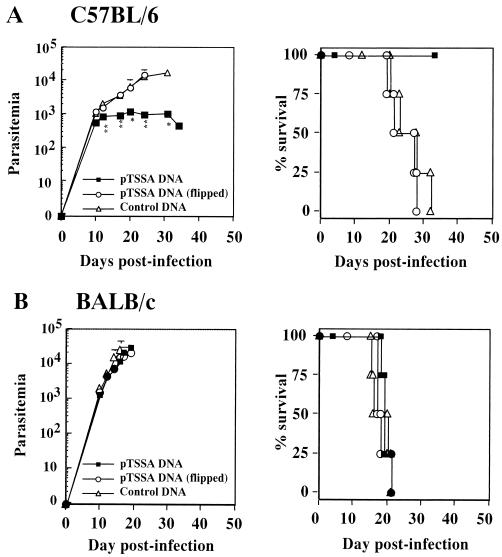
In searching for parasite antigens that could induce protective immunity, we first immunized three strains of mice with plasmid DNA expressing either DHOD or TSSA gene isolated from T. cruzi Tulahuen strain (10). As shown in Fig. 1, the immunization with pDHOD did not protect C57BL/6 (H-2b) mice against lethal T. cruzi infection. Similar results were obtained when BALB/c (H-2d) or C3H/Hej (H-2k) mice were immunized and challenged with T. cruzi in the same experimental protocol (data not shown). All mice manifested a typical course of acute infection, with progressively increasing parasitemia and death ca. 2 to 3 weeks postinfection. The immunization with pTSSA protected all C57BL/6 mice from lethal T. cruzi infection by significantly suppressing the parasitemia (Fig. 2A). However, BALB/c (Fig. 2B) or C3H/Hej mice (data not shown) were not protected by the same vaccination regimen, suggesting that the pTSSA-induced protective immunity might be major histocompatibility complex (MHC) restricted.
FIG. 1.
Immunization with pDHOD encoding T. cruzi DHOD did not protect mice from lethal T. cruzi infection. C57BL/6 mice were i.m. injected with 100 μg of pDHOD (▪) dissolved in 50 μl of PBS into the right hind leg quadriceps four times every 10 days. Control mice were injected with pCMV carrying flipped DHOD gene (○) or with empty pCMV vector (▵). Four mice in each group were challenged with 5,000 T. cruzi blood-form trypomastigotes 11 days after the last immunization. The number of parasites in 5 μl of peripheral blood (parasitemia) (A) was determined periodically, and survival was monitored daily (B).
FIG. 2.
Immunization with pTSSA encoding T. cruzi trans-sialidase surface antigen protected C57BL/6 mice from lethal T. cruzi infection. C57BL/6 (A) or BALB/c (B) mice were i.m. injected with 100 μg of pTSSA (▪) dissolved in 50 μl of PBS into the right hind leg quadriceps four times every 10 days. Control mice were injected with pCMV carrying flipped TSSA gene (○) or with empty pCMV vector (▵). Four mice in each group were challenged with 5,000 T. cruzi blood-form trypomastigotes 10 days after the last immunization. The number of parasites in 5 μl of peripheral blood (parasitemia) was determined periodically, and survival was monitored daily. ✽, P < 0.05 compared to pCMV-immunized mice; ✽✽, P < 0.01 compared to pCMV-immunized mice. The percent survival of pTSSA-immunized C57BL/6 mice was significantly different from that of the other group of mice (P < 0.05).
Vaccine efficacy using pTSSA immunization requires the presence of T cells.
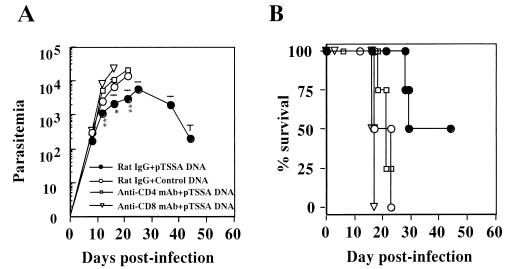
We postulated that the protective immunity conferred by the pTSSA immunization was T cell mediated, as it appeared to be MHC restricted. To determine which effector cells exert the protective immune responses, C57BL/6 mice immunized with pTSSA were treated with anti-CD4 or anti-CD8 monoclonal antibody before the lethal T. cruzi infection in order to deplete CD4+ or CD8+ T cells. The depletion of either CD4+ or CD8+ T cells abolished the suppression of parasitemia (Fig. 3A) and the protection against lethal infection (Fig. 3B) in the pTSSA-immunized mice, indicating the crucial contributions of both CD4+ T cells and CD8+ T cells to the pTSSA-induced protective immunity. These results are consistent with previous studies indicating the pivotal role of CD8+ T cells in resolving acute T. cruzi infection (34, 37, 42, 43, 44) and the critical role of the CD4+ T cells in the full development of CD8+-T-cell responses against intracellular parasites (2, 6, 40).
FIG. 3.
Depletion of CD8+ or CD4+ T cells abolished the protective immunity elicited by pTSSA immunization. C57BL/6 mice were i.m. injected with 100 μg of pTSSA dissolved in 50 μl of PBS into the right hind leg quadriceps four times every 10 days. These mice were i.p. injected with 1 mg of control rat IgG (•), anti-CD8 MAb (▿), or anti-CD4 MAb (□) for three consecutive days until the day of challenge infection. As a control, mice were immunized with empty pCMV vector and treated with 1 mg of control rat IgG (○). Four mice in each group were challenged with 5,000 T. cruzi blood-form trypomastigotes 20 days after the last immunization. The number of parasites in 5 μl of peripheral blood (parasitemia) was determined periodically (A), and survival was monitored daily (B). ✽, P < 0.05 compared to pCMV-immunized, rat IgG-treated mice; ✽✽, P < 0.01 compared to pCMV-immunized, rat IgG-treated mice. The percent survival of pTSSA-immunized, rat IgG-treated C57BL/6 mice was significantly different from that of pCMV-immunized, rat IgG-treated mice (P < 0.05).
Identification of CD8+-T-cell-inducing epitope, ANYNFTLV on TSSA.
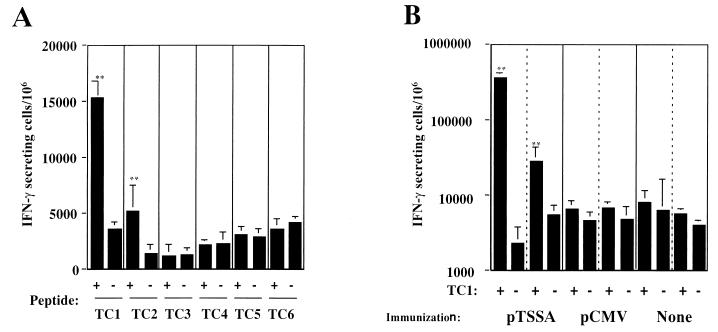
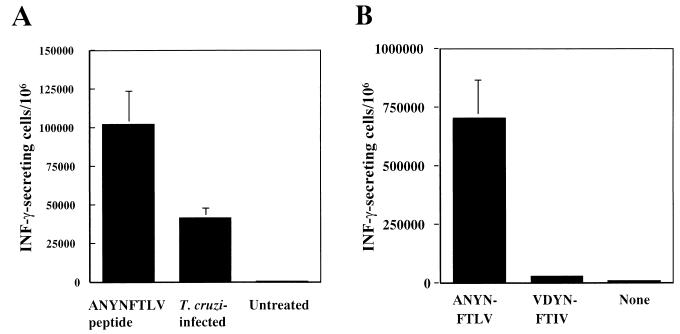
We next searched for the CD8+-T-cell epitope of TSSA. Six peptides which contained the potential H-2Kb- or H-2Db-binding motifs (32) were selected from the TSSA amino acid sequence. Splenocytes isolated from C57BL/6 mice, which had healed from nonlethal T. cruzi infection, were stimulated with each peptide twice in vitro and then subjected to ELISPOT assay against respective peptides. As shown in Fig. 4A, the TC1 peptide, ANYNFTLV (536 to 543), induced a high frequency of IFN-γ-secreting cells. Although the TC2 peptide (QLYHFANYNFTLV; positions 531 to 543) was also effective, we assumed that the effect of TC2 might be derived from the sequence of TC1, which was included in the sequence of TC2. CD8+-T-cell response against the TC1 peptide was also detected in splenocytes derived from the pTSSA-immunized mice but not in those from control vector-immunized mice or naive mice (Fig. 4B). These results suggested that the ANYNFTLV peptide represents a CD8+-T-cell epitope recognized by CD8+ T cells in T. cruzi-infected or pTSSA-immunized C57BL/6 mice.
FIG. 4.
Identification of an H-2Kb-restricted antigenic peptide of TSSA. (A) The following six peptides that contained possible H-2Kb- and H-2Db-binding motif were selected from the TSSA amino acid sequence: TC1 (ANYNFTLV; positions 536 to 543), TC2 (QLYHFANYNFTLV, positions 531 to 543), TC3 (GFNPNKAPI; positions 684 to 692), TC4 (SEDANNNKI; positions 173 to 181), TC5 (SRHLFYSAML 2 to 11), and TC6 (QCKKNGESDIFTGV; positions 77 to 84). Splenocytes from C57BL/6 mice that had been infected with nonlethal dose of T. cruzi 4 months before were stimulated with irradiated EL-4 pulsed with each of these peptides twice at 1-week intervals and then subjected to ELISPOT assay in the presence of irradiated EL-4 cells pulsed with or without the respective peptide. The number of IFN-γ-secreting cells was determined 24 h later. Data are indicated as the mean ± the standard deviation (SD) of triplicate wells. ✽✽, P < 0.01 compared to the data of respective peptide un-pulsed EL-4 cells. (B) Two C57BL/6 mice were i.m. injected with 100 μg of pTSSA into the right hind leg quadriceps. As controls, two mice were immunized with empty pCMV vector, and other two were left untreated. Ten days later, splenocytes from individual mice were stimulated with irradiated EL-4 cells pulsed with TC1 for 1 week, restimulated again for an additional week and then subjected to ELISPOT assay in the presence of irradiated EL-4 pulsed with or without TC1. The number of IFN-γ-secreting cells was determined 24 h later. Data are indicated as the mean ± the SD of triplicate wells for two individual mice in each group. ✽✽, P < 0.01 compared to the data of peptide unpulsed EL-4 cells.
ANYNFTLV-specific T-cell line could recognize T. cruzi infected cells but could not recognize a sequence VDYNFTIV.
We next established CD8+-T-cell line from T. cruzi-infected C57BL/6 splenocytes by repeated in vitro stimulation with the ANYNFTLV peptide. This T-cell line could recognize T. cruzi-infected EL-4 cells in ELISPOT assay, indicating that the ANYNFTLV peptide could be presented via the MHC class I pathway on T. cruzi-infected cells (Fig. 5A). We sought to determine whether the ANYNFTLV-specific CD8+ T cells were cross-reactive with an H-2Kb-restricted T-cell epitope, VDYNFTIV, which was identified on TSSA from different strains of T. cruzi (47). As shown in Fig. 5B, the ANYNFTLV-specific T cells did not recognize the VDYNFTIV peptide. The epitope VDYNFTIV was indicated to be well conserved among several T. cruzi strains, since the T-cell line specific for this epitope could lyse target cells infected with several strains of T. cruzi (47). However, this may not be always true for particular T. cruzi strains such as Tulahuen we used in this study. Our result suggested that we should take the antigenic diversity of each parasite strain into consideration for future vaccine development.
FIG. 5.
A T-cell line specific for ANYNFTLV peptide could recognize T. cruzi-infected cells but was not cross-reactive with VDYNFTIV peptide. (A) A ANYNFTLV-specific CD8+-T-cell line was established as described in Materials and Methods and subjected to ELISPOT assay in the presence of T. cruzi-infected EL-4 cells. ANYNFTLV-pulsed, EL-4 cells were used as a positive control, and untreated EL-4 cells were used as a negative control. The number of IFN-γ-secreting cells was determined 24 h later. Data are indicated as the mean ± the SD of triplicate wells. (B) ANYNFTLV-specific CD8+-T-cell line was subjected to ELISPOT assay in the presence of irradiated EL-4 cells pulsed with or without ANYNFTLV or VDYNFTIV peptide. The number of IFN-γ-secreting cells was determined 24 h later. Data are indicated as the mean ± the SD of triplicate wells.
The CD8+-T-cell epitope, ANYNFTLV, which we identified in this study was incidentally a homologous amino acid of VDYNFTIV, another H-2Kb-restricted CD8+-T-cell epitope identified by Wizel et al. (47). Other homologous amino acids which have been reported so far are; ANHAFTLV in Y strain (nucleotide accession no. D50684), ANHAFTVV in RAstrain (L38456), RNWNFTLH in CL strain (AF051695), VDQNFTLV in Silvio strain (L13844), and LSHNFTLV in G strain (L11287). These peptides closely resemble one another in sequence, and a question arises as to whether these peptides form a network to inhibit or diffuse the immune response to the trans-sialidase gene product. However, the affinity of individual specific peptide to MHC molecule differs markedly, since we demonstrated in Fig. 5B that the ANYNFTLV-specific T-cell line could not recognize the sequence VDYNFTIV. In this respect, we think that T-cell activation will be affected minimally or will not be affected at all by the presence of similar amino acid sequences.
Passive transfer of ANYNFTLV-specific T-cell line protected C57BL/6 mice from lethal T. cruzi infection.
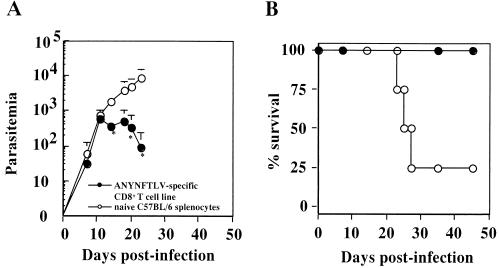
To test whether the CD8+ T cells specific for the ANYNFTLV epitope could be responsible for the protection against lethal T. cruzi infection, we adoptively transferred the ANYNFTLV-specific T-cell line into naive C57BL/6 mice and then challenged them with a lethal dose of T. cruzi. The adoptive transfer of ANYNFTLV-specific T-cell line, but not the same number of naive splenocytes, protected all mice against the lethal infection, with marked suppression of parasitemia (Fig. 6). These results suggested that the CD8+-T-cell responses against the ANYNFTLV epitope could be at least partially responsible for the protective immunity conferred by the pTSSA vaccination. Although we evaluated the induction of ANYNFTLV-specific CD8+ T cells only by ELISPOT assay (assessed by IFN-γ secretion) in our study, several reports strongly suggested that both the cytolytic function and the secretion of IFN-γ from CD8+ T cells are important for resolving infections of intracellular pathogens, including T. cruzi (28). Therefore, we believe that the adoptively transferred ANYNFTLV-specific T-cell line also exerted both functions to resolve the infection.
FIG. 6.
Adoptive transfer of ANYNFTLV-specific CD8+-T-cell line protected naive C57BL/6 mice from lethal T. cruzi infection. Four naive C57BL/6 mice were intravenously injected with 5 × 107 ANYNFTLV-specific CD8+ T cells on days 0, 7, and 14 (•). As a control, another group of four mice received the same number of naive C57BL/6 splenocytes (○). All mice were i.p. inoculated with 1,000 T. cruzi blood-form trypomastigotes on day 0. The number of parasites in 5 μl of peripheral blood (parasitemia) was determined periodically (A), and survival was monitored daily (B). ✽, P < 0.05 compared to naive splenocyte-transferred mice. Similar results were obtained in two independent experiments.
Coadministration of IL-12 gene with pTSSA improved the vaccine efficacy as determined by ELISPOT and protection assays.
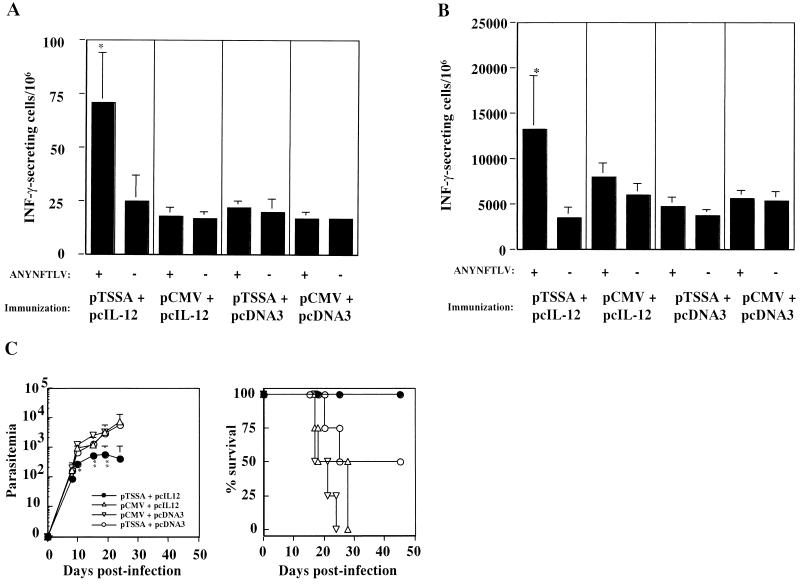
To improve the efficacy of pTSSA vaccination, we next tested the adjuvant effect of the IL-12-expressing vector (pcIL-12) coadministrated with pTSSA. Development of ANYNFTLV-specific T cells in the spleen was measured by ELISPOT assay to monitor the CD8+-T-cell responses to TSSA. Although the immunization of pTSSA with control pcDNA3 twice did not induce the ANYNFTLV-specific T cells, as estimated before (Fig. 7A) or after (Fig. 7B) in vitro culture with the peptide, the coimmunization of pTSSA with pcIL-12 significantly induced the ANYNFTLV-specific T cells in the spleen which could be detected even before the in vitro amplification (P < 0.05). When challenged with lethal dose of T. cruzi, all of the mice immunized twice with pTSSA plus pcIL-12 survived with marked suppression of parasitemia, whereas no mice immunized with pCMV plus pcDNA3 survived (P < 0.05). Although a half of the mice immunized twice with pTSSA plus pcDNA3 survived, statistical analysis revealed no significant difference compared to control groups. These results indicated a potential utility of IL-12 gene as an adjuvant to induce the protective CD8+-T-cell responses against T. cruzi by TSSA gene vaccination. We believe that other immune effector cell populations such as CD4+ T cells could be also immunologically enhanced by IL-12 administration. We do not intend to attribute the effect of IL-12 for enhancing protective immune responses solely to CD8+ T cells; however, considering the central roles of this cell population in resolving acute infection, we think that the augmentation of CD8+-T-cell responses would have the biggest impact on conferring protective immunity against T. cruzi infection.
FIG. 7.
Coimmunization of pTSSA with pcIL-12 enhanced the induction of ANYNFTLV-specific CD8+ T cells and improved the protection from lethal T. cruzi infection. Three C57BL/6 mice in each group were i.m. injected into the right hind leg quadriceps with 100 μg each of pTSSA plus pcIL-12, pCMV plus pcIL-12, pTSSA plus pcDNA3, or pCMV plus pcDNA3 twice at 10-day intervals. Ten days after the second immunization, splenocytes were immediately subjected to ELISPOT assay in the presence of irradiated EL-4 cells pulsed with or without ANYNFTLV peptide (A), or stimulated with ANYNFTLV-pulsed EL-4 cells for 1 week and then subjected to the ELISPOT assay (B). The number of IFN-γ-secreting cells was counted 24 h later. Data are indicated as the mean ± the SD of three mice in each group. ✽, P < 0.05 compared to the data of peptide unpulsed EL-4 cells. (C) Four C57BL/6 mice in each group were immunized with pTSSA plus pcIL12 (•), pTSSA plus pcDNA3 (○), pCMV plus pcIL12 (▵), or pCMV plus pcDNA3 (▿) as in panel A and then challenged with 5,000 T. cruzi blood-form trypomastigotes 14 days after the second immunization. The number of parasites in 5 μl of peripheral blood (parasitemia) was counted periodically, and survival was monitored daily. ✽, P < 0.05 compared to pCMV plus pcDNA3-immunized mice; ✽✽, P < 0.01 compared to pCMV plus pcDNA3-immunized mice. The percent survival of pTSSA plus pcIL-12-immunized C57BL/6 mice was significantly different from that of pCMV plus pcDNA3-immunized mice (P < 0.05), while that of pTSSA plus pcDNA3-immunized mice was not. Similar results were obtained in two independent experiments.
The potent capacity of IL-12 gene coadministration to enhance the specific CD8+-T-cell responses was presumably mediated by the transformation of local myocytes or dendritic cells (DCs) (1, 14). The DCs transformed with both pTSSA and pcIL-12 might directly interact with CD8+ T cells or act indirectly on CD8+ T cells via CD4+-T-cell activation in regional lymph nodes. Another possibility would be that pTSSA- and pcIL-12-transformed local myocytes might be lysed by natural killer cells that were activated by the transformed myocyte-secreting IL-12. This process might accelerate the cross-priming of DCs (14), leading to the stronger induction of epitope-specific CD8+-T-cell responses. In addition to these possible mechanisms, transformed-cell secreted IL-12 might induce the proliferation of whole-cell population, including T cells in vivo. This might result in the expansion of precursor CD8+ T cells, leading to the enhanced epitope-specific T-cell proliferation, although the proportion of CD8+ T cells was reported not to increase by IL-12 administration (3). Precise immunological mechanisms for IL-12 adjuvant effect will have to be clarified in order to enable the rational design of vaccination strategies. Although we tested only IL-12 here to evaluate its potential to enhance CD8+-T-cell-mediated immunity against intracellular parasite, other costimulatory molecules (8, 19) and cytokines (30) are also worth testing in future studies.
Acknowledgments
This work was supported in part by the Ohyama Health Foundation to Y.M. and also in part by a grant for the Research For The Future Program (JSPS-RFTF-97L00701).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Apostolopoulos, V., and M. Plebanski. 2000. The evolution of DNA vaccines. Curr. Opin. Mol. Ther. 2:441-447. [PubMed] [Google Scholar]
- 2.Bennett, S. R., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478-480. [DOI] [PubMed] [Google Scholar]
- 3.Bloom, E. T., and J. A. Horvath. 1994. Cellular and molecular mechanisms of the IL-12-induced increase in allospecific murine cytolytic T-cell activity: implications for the age-related decline in CTL. J. Immunol. 152:4242-4254. [PubMed] [Google Scholar]
- 4.Bone, G. J., and M. Steinert. 1956. Isotopes incorporated in the nucleic acids of Trypanosoma mega. Nature 178:308-309. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho, L. H., J. C. R. Hafalla, and F. Zavala. 2001. ELISPOT assay to measure antigen-specific murine CD8+-T-cell responses. J. Immunol. Methods 252:207-218. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho, L. H., G. Sano, J. C. R. Hafalla, Morrot, A., M. A. C. Lafaille, and F. Zavala. 2002. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 8:166-170. [DOI] [PubMed] [Google Scholar]
- 7.Chagas, C. 1909. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., agente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz 1:159-218. [Google Scholar]
- 8.Croft, M., and C. Dubey. 1997. Accessory molecule and costimulation requirements for CD4 T cell response. Crit. Rev. Immunol. 17:89-118. [DOI] [PubMed] [Google Scholar]
- 9.Fujimura, A. E., S. S. Kinoshita, V. L. Pereira-Chioccola, and M. M. Rodrigues. 2001. DNA sequences encoding CD4+ and CD8+ T-cell epitopes are important for efficient protective immunity induced by DNA vaccination with a Trypanosoma cruzi gene. Infect. Immun. 69:5477-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao, G., T. Nara, J. Nakajima-Shimada, and T. Aoki. 1999. Novel organization and sequences of five genes encoding all six enzymes for de novo pyrimidine biosynthesis in Trypanosoma cruzi. J. Mol. Biol. 285:149-161. [DOI] [PubMed] [Google Scholar]
- 11.Gherardi, M. M., J. C. Ramirez, and M. Esteban. 2001. Towards a new generation of vaccines: the cytokine IL-12 as an adjuvant to enhance cellular immune responses to pathogens during prime-booster vaccination regimens. Histopathology 16:655-667. [DOI] [PubMed] [Google Scholar]
- 12.Gras-Masse, H. 2001. Single-chain lipopeptide vaccines for the induction of virus-specific cytotoxic T-cell responses in randomly selected populations. Mol. Immunol. 38:423-431. [DOI] [PubMed] [Google Scholar]
- 13.Gurunathan, S., C. Prussin, D. L. Sacks, and R. A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409-1415. [DOI] [PubMed] [Google Scholar]
- 14.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 15.Hunter, C. A., T. Slifer, and F. Araujo. 1996. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor alpha and gamma interferon. Infect. Immun. 64:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jager, D., E. Jager, and A. Knuth. 2001. Vaccination for malignant melanoma: recent developments. Oncology 60:1-7. [DOI] [PubMed] [Google Scholar]
- 17.Kirchhoff, L. V. 1990. Trypanosoma species (American trypanosomiasis, Chagas disease): biology of trypanosomes, p. 2077-2084. In G. L. Mandell, R. G. Douglas, Jr., and J. E. Bennett (ed.), Principles and practice of infectious diseases, 3rd ed. Churchill Livingstone, New York, N.Y.
- 18.Kirchhoff, L.V. 1993. American trypanosomiasis (Chagas' disease): a tropical disease now in the United States. N. Engl. J. Med. 329:639-644. [DOI] [PubMed] [Google Scholar]
- 19.Kobata, T., M. Azuma, H. Yagita, and K. Okumura. 2000. Role of costimulatory molecules in autoimmunity. Rev. Immunogenet. 2:74-80. [PubMed] [Google Scholar]
- 20.Low, H. P., M. A. Santos, B. Wizel, and R. L. Tarleton. 1998. Amastigote surface proteins of Trypanosoma cruzi are targets for CD8+ CTL. J. Immunol. 160:1817-1823. [PubMed] [Google Scholar]
- 21.Marchal, I., M. Cerutti, A. M. Mir, S. Juliant, G. Devauchelle, R. Cacan, and A. Verbert. 2001. Expression of a membrane-bound form of Trypanosoma cruzi trans-sialidase in baculovirus-infected insect cells: a potential tool for sialylation of glycoproteins produced in the baculovirus-insert cells system. Glycobiology 11:593-603. [DOI] [PubMed] [Google Scholar]
- 22.Miyahira, Y., and J. A. Dvorak. 1994. Kinetoplastidae display naturally occurring ancillary DNA-containing structures. Mol. Biochem. Parasitol. 65:339-349. [DOI] [PubMed] [Google Scholar]
- 23.Miyahira, Y., K. Murata, D. Rodriguez, J. R. Rodriguez, M. Esteban, M. M. Rodrigues, and F. Zavala. 1995. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J. Immunol. Methods 181:45-54. [DOI] [PubMed] [Google Scholar]
- 24.Miyahira, Y., A. Garcia-Sastre, D. Rodriguez, J. R. Rodriguez, K. Murata, M. Tsuji, P. Palese, M. Esteban, F. Zavala, and R. S. Nussenzweig. 1998. Recombinant viruses expressing a human malaria antigen can elicit potentially protective immune CD8+ responses in mice. Proc. Natl. Acad. Sci. USA 95:3954-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyahira, Y., S. Kobayashi, T. Takeuchi, T. Kamiyama, T. Nara, J. Nakajima-Shimada, and T. Aoki. 1999. Induction of CD8+ T cell-mediated protective immunity against Trypanosoma cruzi. Int. Immunol. 11:133-141. [DOI] [PubMed] [Google Scholar]
- 26.Mortara, L., H. Gras-Masse, C. Rommens, A. Venet, J. G. Guillet, and I. Bourgault-Villada. 1999. Type 1 CD4+ T-cell help is required for induction of antipeptide multispecific cytotoxic T lymphocytes by a lipopeptidic vaccine in rhesus macaques. J. Virol. 73:4447-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata, K., A. Garcia-Sastre, M. Tsuji, M. Rodrigues, D. Rodriguez, J. R. Rodriguez, R. S. Nussenzweig, P. Palese, M. Esteban, and F. Zavala. 1996. Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia viruses. Cell. Immunol. 173:96-107. [DOI] [PubMed] [Google Scholar]
- 28.Nickell, S. P., and D. Sharma. 2000. Trypanosoma cruzi: roles for perforin-dependent and perforin-independent immune mechanisms in acute resistance. Exp. Parasitol. 94:207-216. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira-Ferreira, J., Y. Miyahira, G. T. Layton, N. Savage, M. Esteban, D. Rodriguez, J. R. Rodriguez, R. S. Nussenzweig, and F. Zavala. 2000. Immunogenicity of Ty-VLP bearing a CD8+ T cell epitope of the CS protein of P. yoelii: enhanced memory response by boosting with recombinant vaccinia virus. Vaccine 17:1863-1869. [DOI] [PubMed] [Google Scholar]
- 30.Pasquini, S., Z. Xiang, Y. Wang, Z. He, H. Deng, M. Blaszczyk-Thurin, and H. C. Ertl. 1997. Cytokines and costimulatory molecules as genetic adjuvants. Immunol. Cell. Biol. 75:397-401. [DOI] [PubMed] [Google Scholar]
- 31.Prata, A. 2001. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 1:92-100. [DOI] [PubMed] [Google Scholar]
- 32.Rammensee, H. G., T., Friede, and S. Stevanoviic. 1995. MHC ligands and peptide mortifs: first listing. Immunogenetics 41:178-228. [DOI] [PubMed] [Google Scholar]
- 33.Ramshaw, I. A., and A. J. Ramsay. 2000. The prime-boost strategy: exciting prospects for improved vaccination. Immunol. Today 21:163-165. [DOI] [PubMed] [Google Scholar]
- 34.Revelli, S., H. Davila, M. E. Ferro, M. Romero-Piffiguer, O. Musso, J. Valenti, J. Bernabo, E. Falcoff, J. Wietzerbin, and O. Bottasso. 1992. Acute and chronic experimental Trypanosoma cruzi infection in the rat. Response to systemic treatment with recombinant rat interferon-gamma. Microbiol. Immunol. 39:275-281. [DOI] [PubMed] [Google Scholar]
- 35.Rodolfo, M., and M. P. Colombo. 1999. Interleukin-12 as an adjuvant for cancer immunotherapy. Methods 19:114-120. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues, M. M., M. Ribeirao, V. Pereira-Chioccola, L. Renia, and F. Costa. 1999. Predominance of CD4 Th1 and CD8 Tc1 cells revealed by characterization of the cellular immune response generated by immunization with a DNA vaccine containing a Trypanosoma cruzi gene. Infect. Immun. 67:3855-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rottenberg, M. E., A. Riarte, L. Sorrong, J. Altcheh, P. Petray, A. M. Ruiz, and H. Wigzell. 1995. Outcome of infection with different strains of Trypanosoma cruzi in mice lacking CD4 and/or CD8. Immunol. Lett. 45:53-60. [DOI] [PubMed] [Google Scholar]
- 38.Sakai, T., H. Hisaeda, Y. Nakano, H. Ishikawa, Y. Maekawa, K. Ishii, Y. Nitta, J. Miyazaki, and K. Himeno. 2000. Gene gun-mediated delivery of an interleukin-12 expression plasmid protects against infections with the intracellular protozoan parasites Leishmania major and Trypanosoma cruzi in mice. Immunology 99:615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schenkman, S., D. Eichinger, M. E. A. Pereira, and V. Nussenzweig. 1994. Structural and functional properties of Trypanosoma trans-sialidase. Annu. Rev. Microbiol. 48:499-523. [DOI] [PubMed] [Google Scholar]
- 40.Schoenberger, S. P., R. E. Toes, E.I. van der Voort, R. Offringa, and C. J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480-483. [DOI] [PubMed] [Google Scholar]
- 41.Stephenson, J. R. 2001. Genetically modified viruses: vaccines by design. Curr. Pharm. Biotechnol. 2:47-76. [DOI] [PubMed] [Google Scholar]
- 42.Tarleton, R. L. 1990. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J. Immunol. 144:717-724. [PubMed] [Google Scholar]
- 43.Tarleton, R. L., B. H. Koller, A. Latour, and M. Postan. 1992. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356:338-340. [DOI] [PubMed] [Google Scholar]
- 44.Tarleton, R. L., M. J. Grusby, M. Postan, and L. H. Glimcher. 1996. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int. Immunol. 8:13-22. [DOI] [PubMed] [Google Scholar]
- 45.Toda, M., R. L. Martuza, H. Kojima, and S. D. Rabkin. 1998. In situ cancer vaccination: an IL-12 defective vector/replication-competent herpes simplex virus combination induces local and systemic antitumor activity. J. Immunol. 160:4457-4464. [PubMed] [Google Scholar]
- 46.Trinchieri, G. 1998. Immunobiology of interleukin-12. Immunol. Res. 17:269-278. [DOI] [PubMed] [Google Scholar]
- 47.Wizel, B., M. Nunes, and R. L. Tarleton. 1997. Identification of Trypanosoma cruzi trans-sialidase family members as targets of protective CD8+ TC1 responses. J. Immunol. 159:6120-6130. [PubMed] [Google Scholar]
- 48.Wizel, B., N. Garg, and R. L. Tarletion. 1998. Vaccination with trypomastigote surface antigen 1-encoding plasmid DNA confers protection against lethal Trypanosoma cruzi infection. Infect. Immun. 66:5073-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wrighsman, R. A., and J. E. Manning. 2000. Paraflagellar rod proteins administered with alum and IL-12 or recombinant adenovirus expressing IL-12 generates antigen-specific responses and protective immunity in mice against Trypanosoma cruzi. Vaccine 18:1419-1427. [DOI] [PubMed] [Google Scholar]
- 50.Zavala, F., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, R. S. Nussenzweig, and M. Esteban. 2001. A striking property of recombinant poxviruses: efficient inducers of in vivo expansion of primed CD8+ T cells. Virology 280:155-159. [DOI] [PubMed] [Google Scholar]