Abstract
1. Squid giant axons internally perfused with CsF have their Na conductance inactivated due to the low value of the resting potential. When hyperpolarized with voltage clamp to normal values of resting potential, the Na conductance recovers with an exponential time course. The time constant of recovery is of the order of 30 sec at a membrane potential of -70 mV and at 5 degrees C. The recovery from slow inactivation has a Q10 of about 3. 2. The development of inactivation during depolarization is also slow. The time constant varies between 10 and 20 sec at 5 degrees C, depending upon the value of the membrane potential. 3. Slow inactivation is also observed in NaF perfused axons and in intact axons with a low resting potential. 4. Although internal perfusion with pronase (or a purified fraction of this enzymic complex) blocks the fast (h) inactivation of the Na conductance, the slow inactivation remains. The recovery is similar before and after the proteolytic treatment. However, slow inactivation appears to develop faster after enzymic perfusion. 5. Slow inactivation develops without any apparent change in distributed or local membrane surface charge. 6. The experiments suggest that slow inactivation is a general property of the Na conductance as in many other conductance channels in excitable membranes. The experiments can be interpreted by proposing that slow inactivation is a phenomenon independent of fast inactivation, and that pronase somehow accelerates the onset of slow inactivation. 7. An alternative model, in which slow inactivation is coupled to fast inactivation, is proposed. This model is consistent with the results presented here and is very similar to one proposed to explain the frequency response of the sodium currents in Myxicola giant axons (Rudy, 1975, 1978).
Full text
PDF



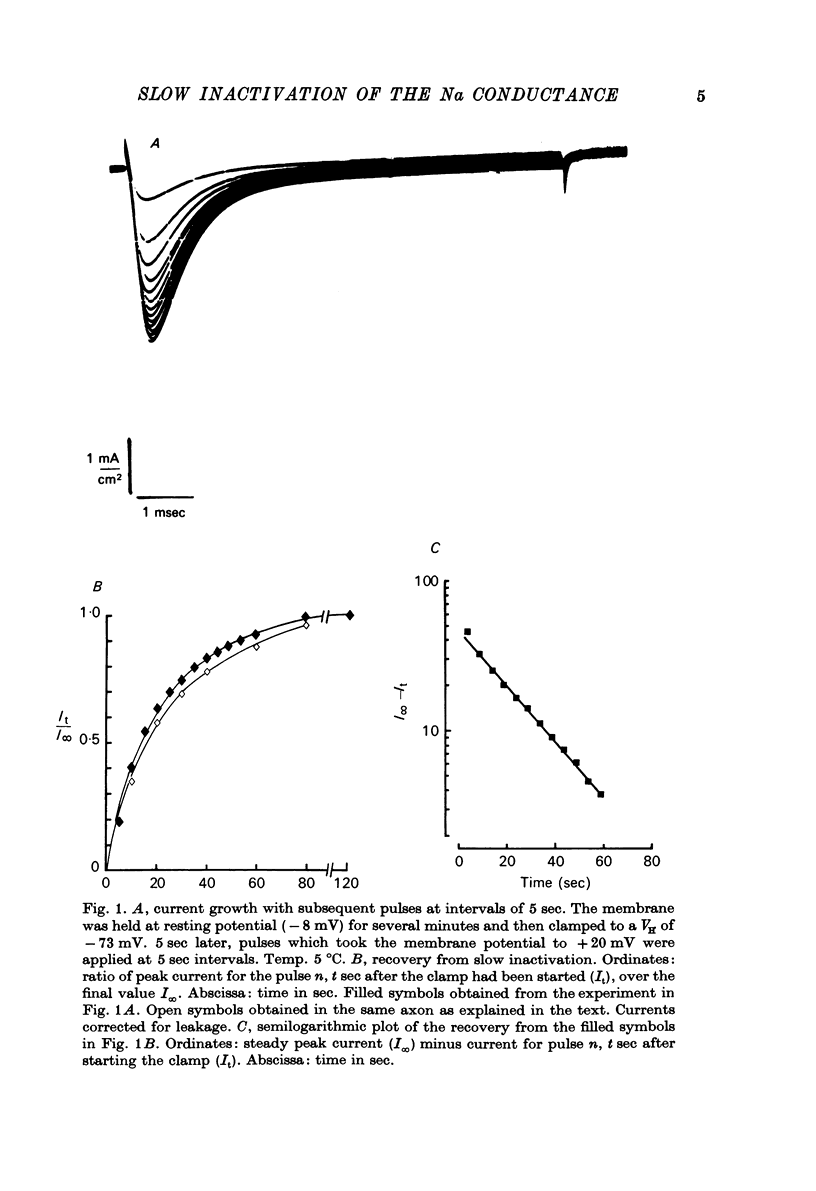


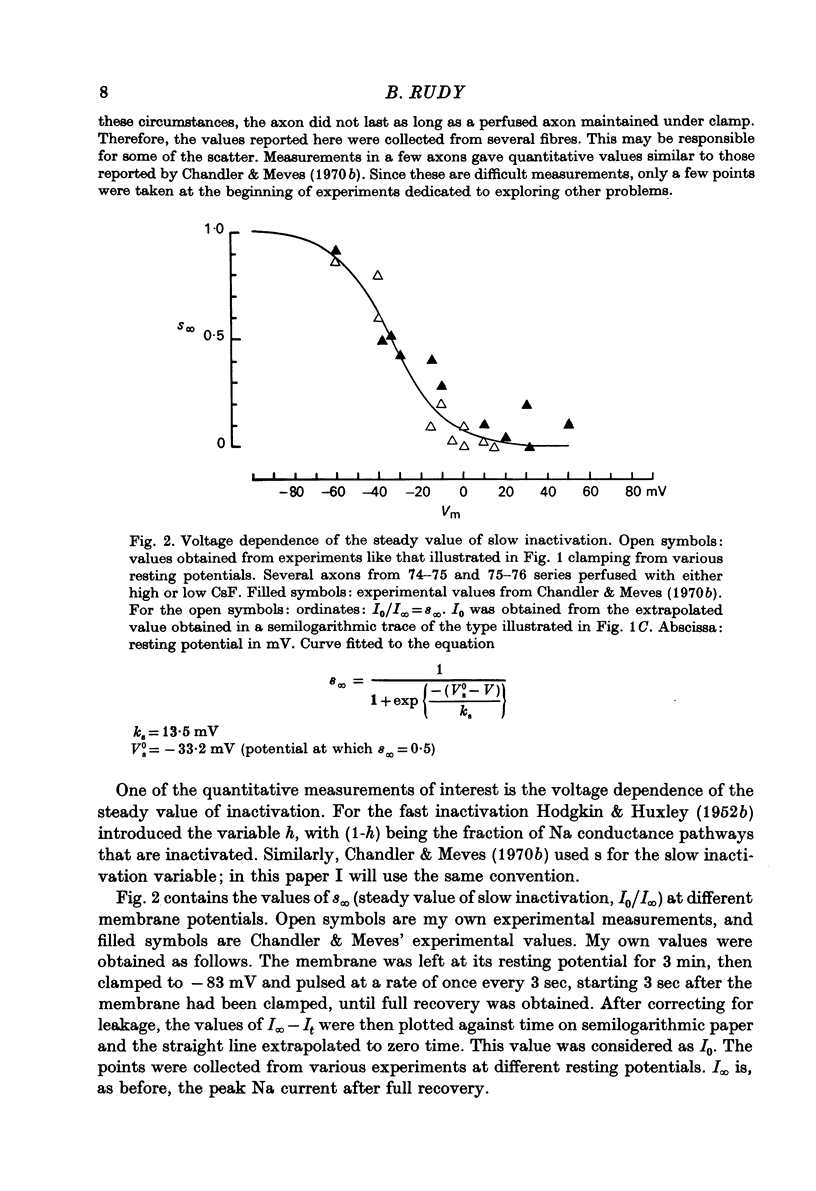
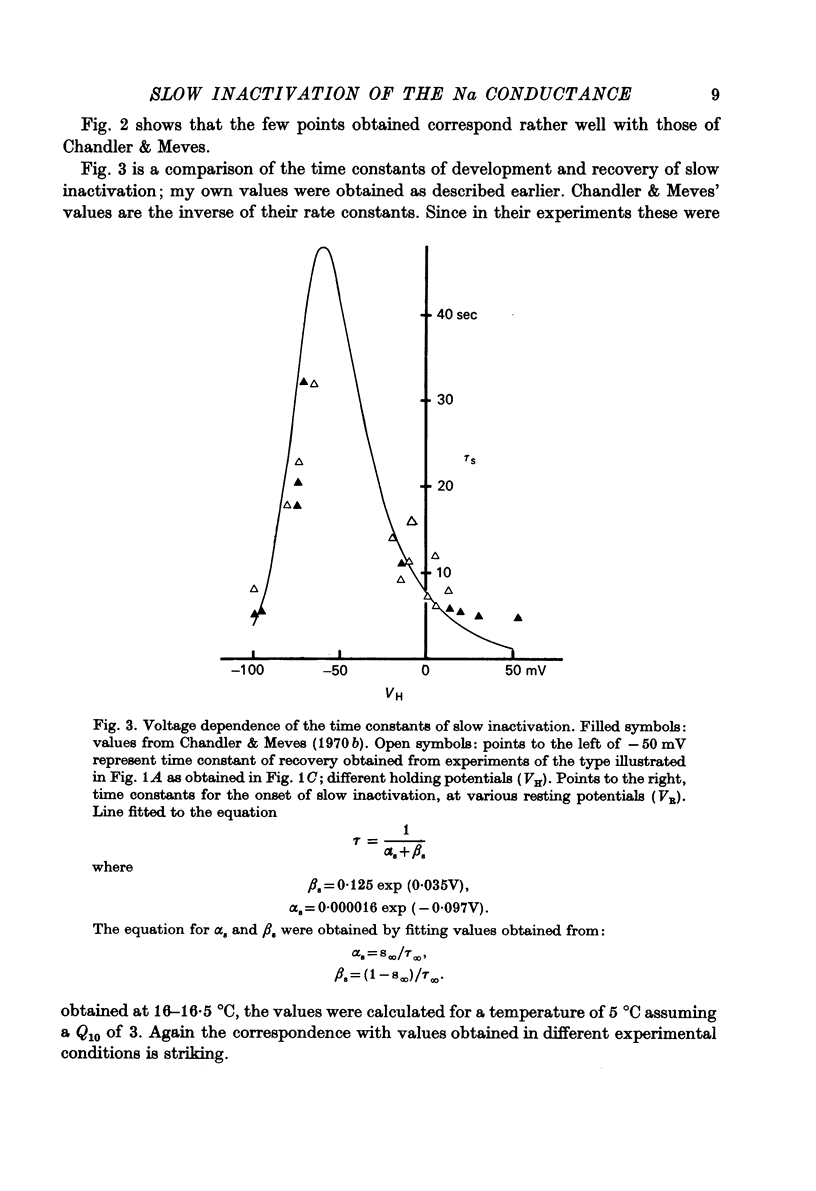
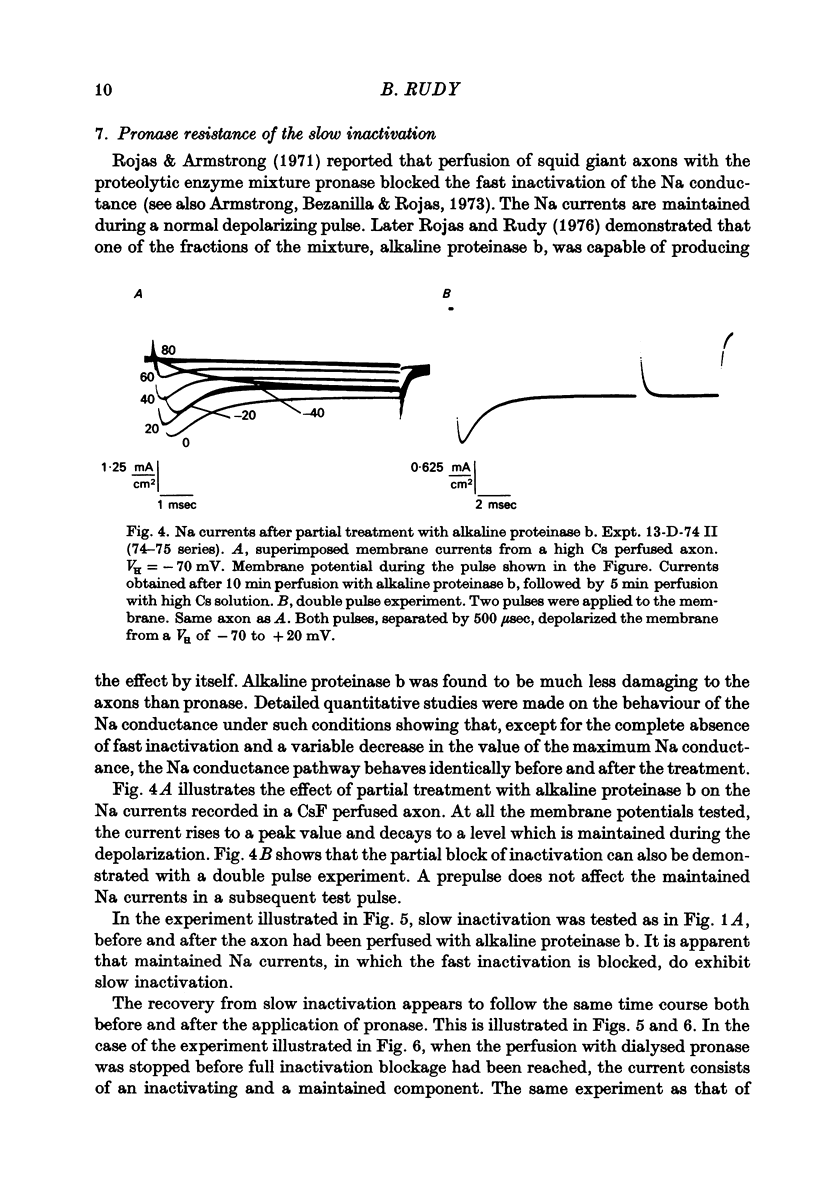
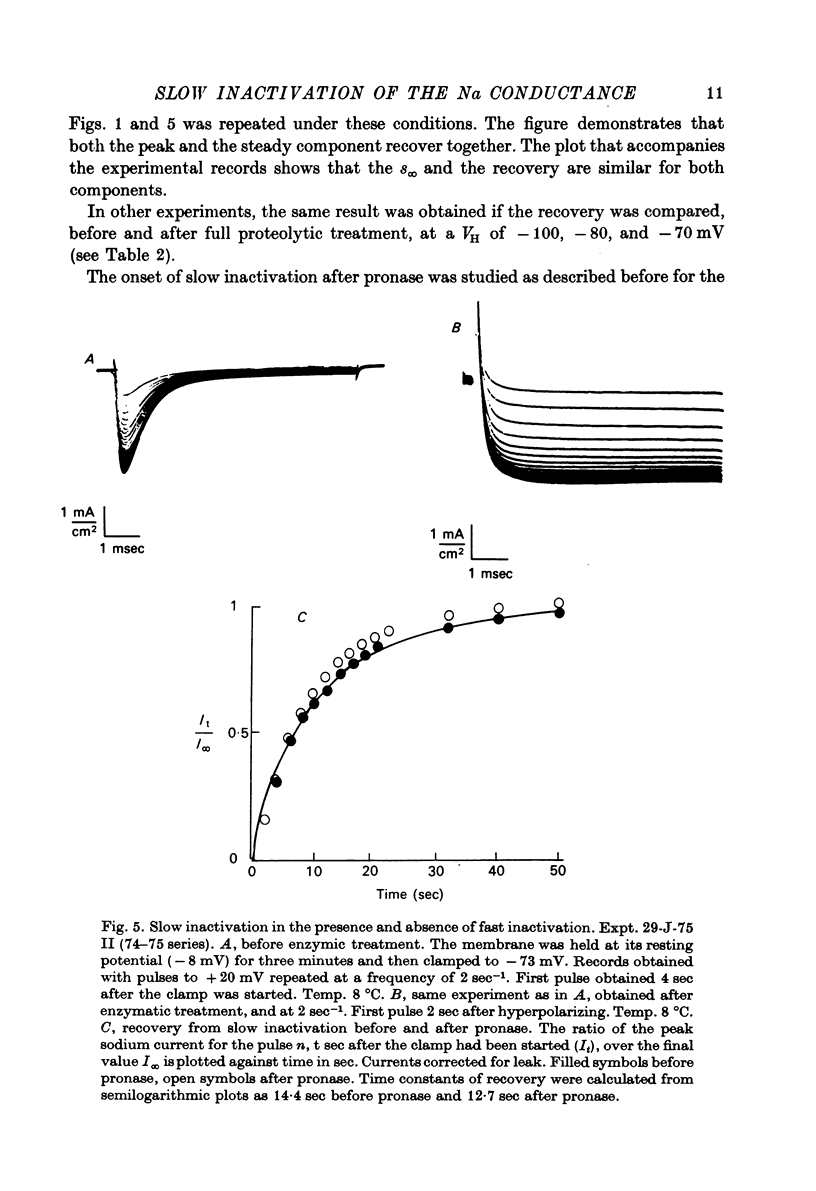
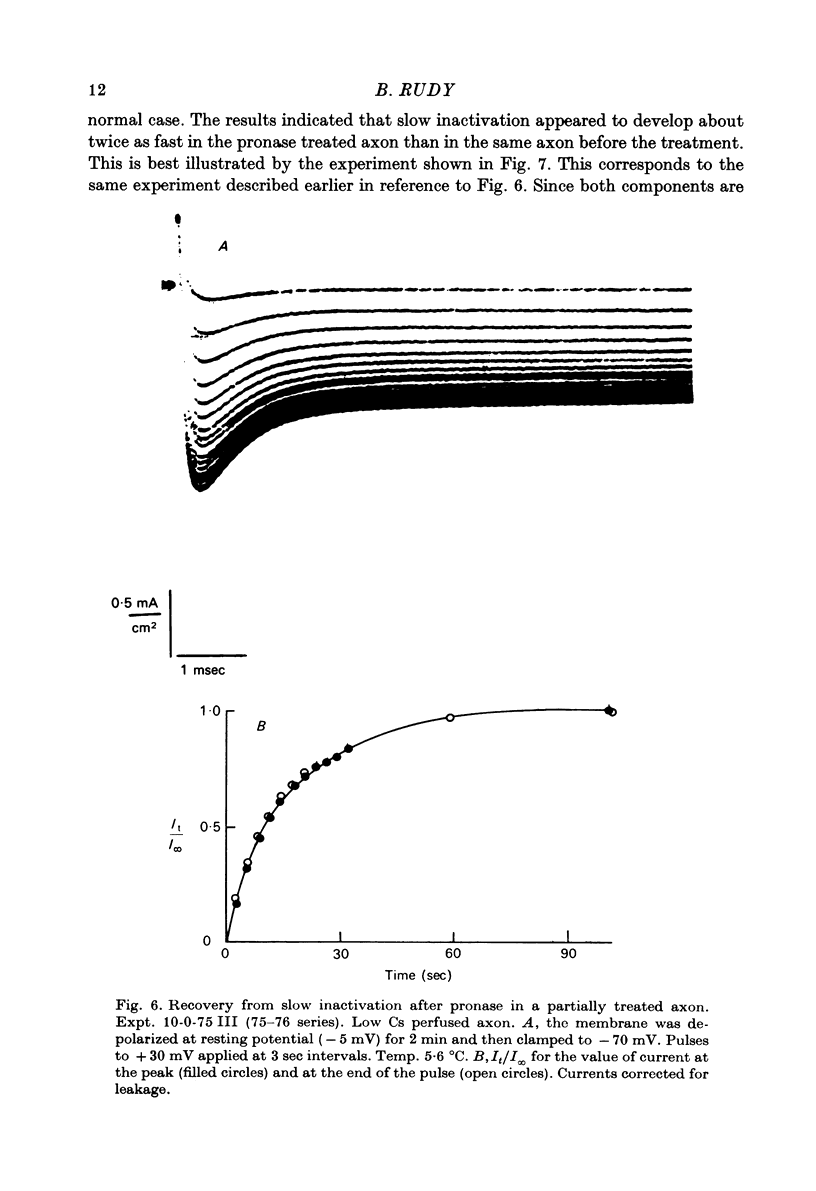
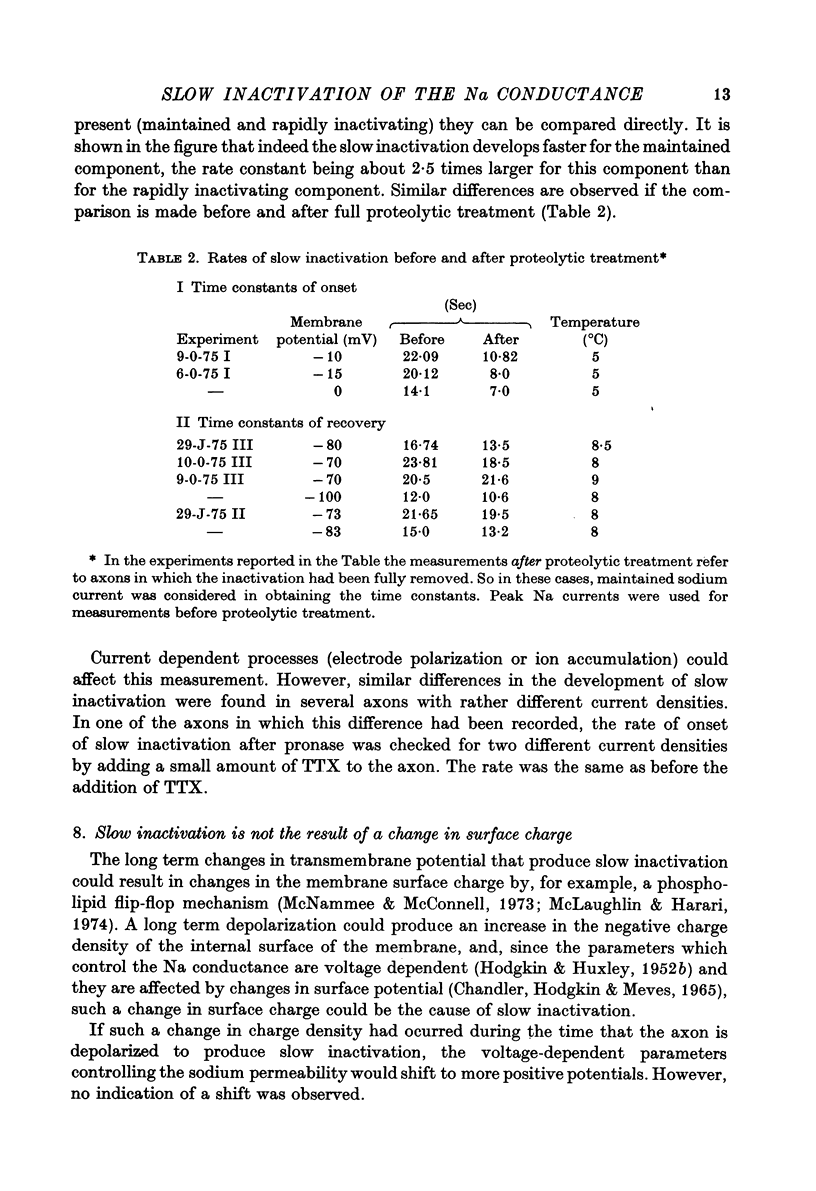
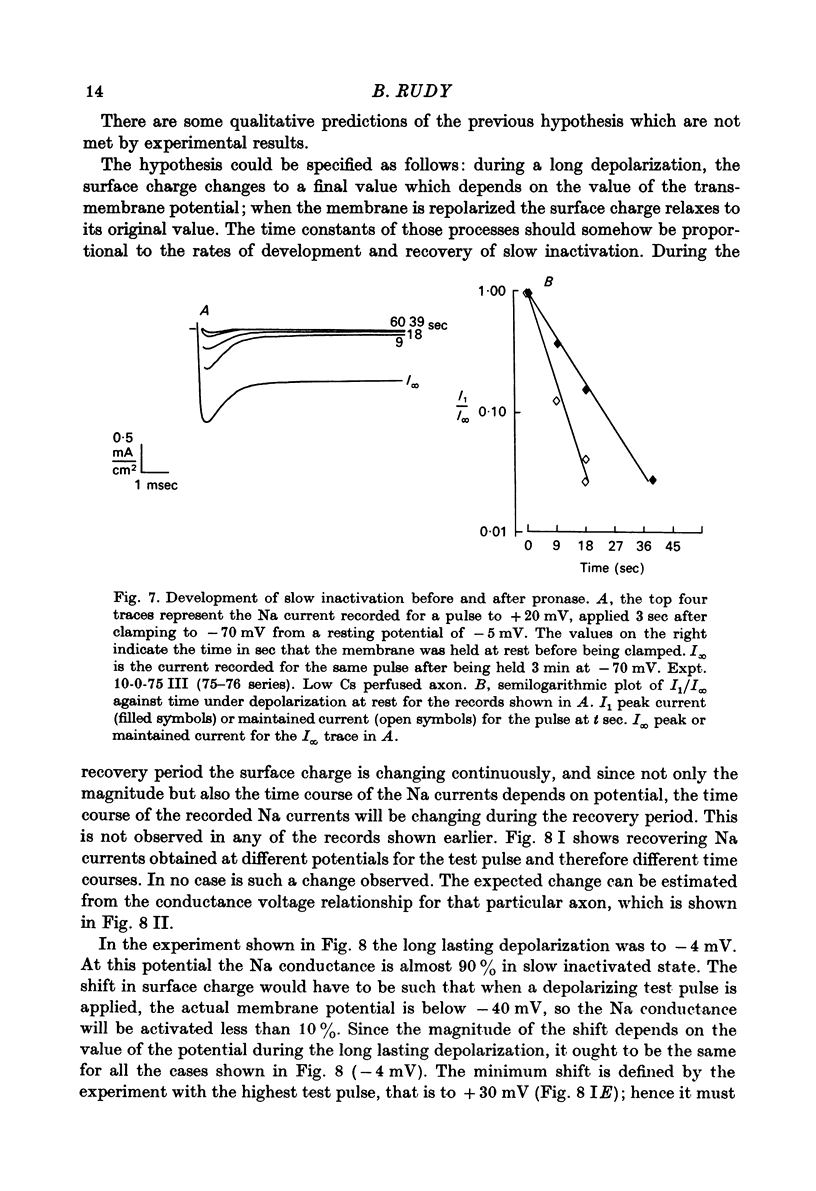





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Palti Y. The effects of external potassium and long duration voltage conditioning on the amplitude of sodium currents in the giant axon of the squid, Loligo pealei. J Gen Physiol. 1969 Nov;54(5):589–606. doi: 10.1085/jgp.54.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Palti Y. The influence of external potassium on the inactivation of sodium currents in the giant axon of the squid, Loligo pealei. J Gen Physiol. 1969 Jun;53(6):685–703. doi: 10.1085/jgp.53.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Senft J. P. Voltage clamp studies on the effect of internal cesium ion on sodium and potassium currents in the squid giant axon. J Gen Physiol. 1966 Nov;50(2):279–293. doi: 10.1085/jgp.50.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Rink T. J. Catecholamine release from bovine adrenal medulla in response to maintained depolarization. J Physiol. 1975 Dec;253(2):593–620. doi: 10.1113/jphysiol.1975.sp011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Gating currents of the sodium channels: three ways to block them. Science. 1974 Feb 22;183(4126):753–754. doi: 10.1126/science.183.4126.753. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Evidence for two types of sodium conductance in axons perfused with sodium fluoride solution. J Physiol. 1970 Dec;211(3):653–678. doi: 10.1113/jphysiol.1970.sp009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Slow changes in membrane permeability and long-lasting action potentials in axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):707–728. doi: 10.1113/jphysiol.1970.sp009300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Sodium and potassium currents in squid axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):623–652. doi: 10.1113/jphysiol.1970.sp009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer R. J., Muller R. U., Finkelstein A. Inactivation of monazomycin-induced voltage-dependent conductance in thin lipid membranes. II. Inactivation produced by monazomycin transport through the membrane. J Gen Physiol. 1976 Jun;67(6):731–748. doi: 10.1085/jgp.67.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Harary H. Phospholipid flip-flop and the distribution of surface charges in excitable membranes. Biophys J. 1974 Mar;14(3):200–208. doi: 10.1016/S0006-3495(74)85907-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee M. G., McConnell H. M. Transmembrane potentials and phospholipid flip-flop in excitable membrane vesicles. Biochemistry. 1973 Jul 31;12(16):2951–2958. doi: 10.1021/bi00740a001. [DOI] [PubMed] [Google Scholar]
- Meves H. The effect of zinc on the late displacement current in squid giant axons. J Physiol. 1976 Jan;254(3):787–801. doi: 10.1113/jphysiol.1976.sp011259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke B., Fox J. M., Drouin H., Schwarz W. Kinetics of the slow variation of peak sodium current in the membrane of myelinated nerve following changes of holding potential or extracellular pH. Biochim Biophys Acta. 1976 Mar 5;426(2):245–257. doi: 10.1016/0005-2736(76)90335-7. [DOI] [PubMed] [Google Scholar]
- Rojas E., Armstrong C. Sodium conductance activation without inactivation in pronase-perfused axons. Nat New Biol. 1971 Feb 10;229(6):177–178. doi: 10.1038/newbio229177a0. [DOI] [PubMed] [Google Scholar]
- Rojas E., Rudy B. Destruction of the sodium conductance inactivation by a specific protease in perfused nerve fibres from Loligo. J Physiol. 1976 Nov;262(2):501–531. doi: 10.1113/jphysiol.1976.sp011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Taylor R. E., Atwater I., Bezanilla F. Analysis of the effects of calcium or magnesium on voltage-clamp currents in perfused squid axons bathed in solutions of high potassium. J Gen Physiol. 1969 Oct;54(4):532–552. doi: 10.1085/jgp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Proceedings: Slow recovery of the inactivation of sodium conductance in Myxicola giant axons. J Physiol. 1975 Jul;249(1):22P–24P. [PubMed] [Google Scholar]
- Schauf C. L., Pencek T. L., Davis F. A. Slow sodium inactivation in Myxicola axons. Evidence for a second inactive state. Biophys J. 1976 Jul;16(7):771–778. doi: 10.1016/S0006-3495(76)85727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J. R., Vogel W. Potassium inactivation in single myelinated nerve fibres of Xenopus laevis. Pflugers Arch. 1971;330(1):61–73. doi: 10.1007/BF00588735. [DOI] [PubMed] [Google Scholar]
- Standen N. B. Properties of a calcium channel in snail neurones. Nature. 1974 Jul 26;250(464):340–342. doi: 10.1038/250340a0. [DOI] [PubMed] [Google Scholar]
- TASAKII, WATANABE A., TAKENAKA T. Resting and action potential of intracellularly perfused squid giant axon. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1177–1184. doi: 10.1073/pnas.48.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]


