Abstract
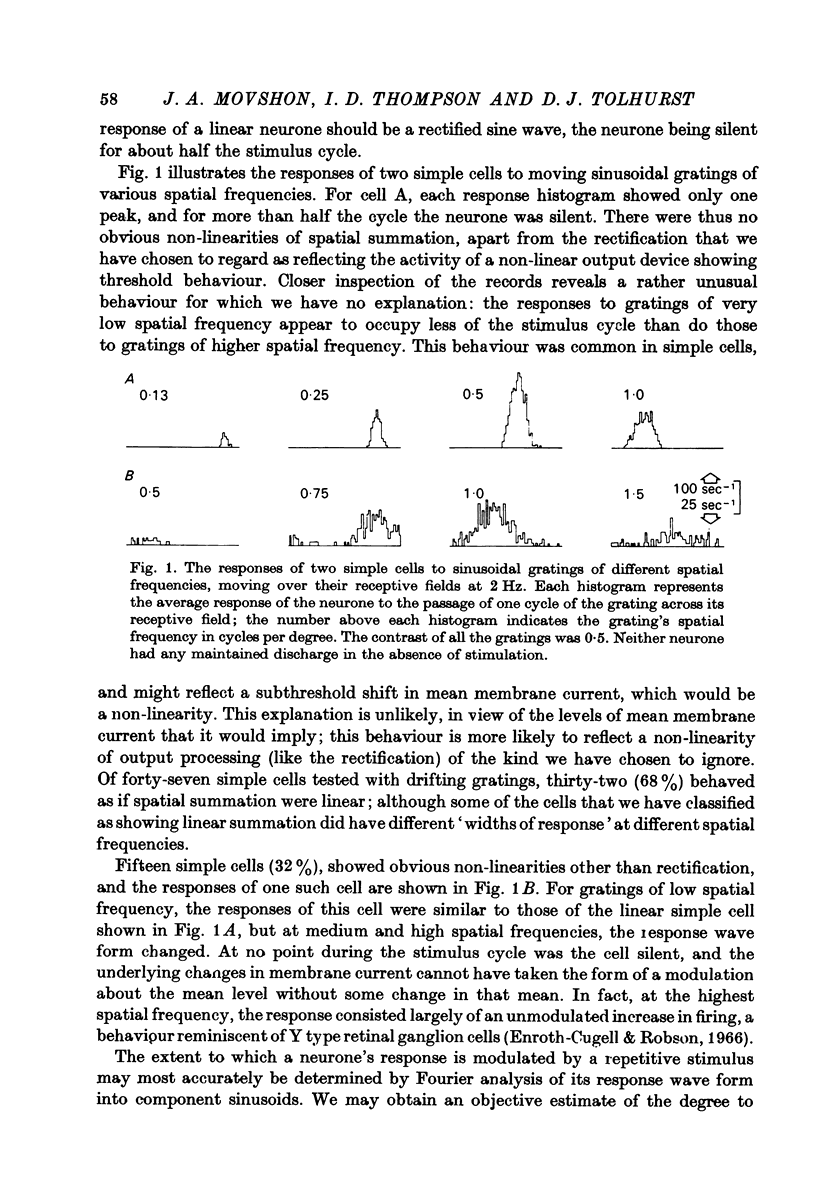
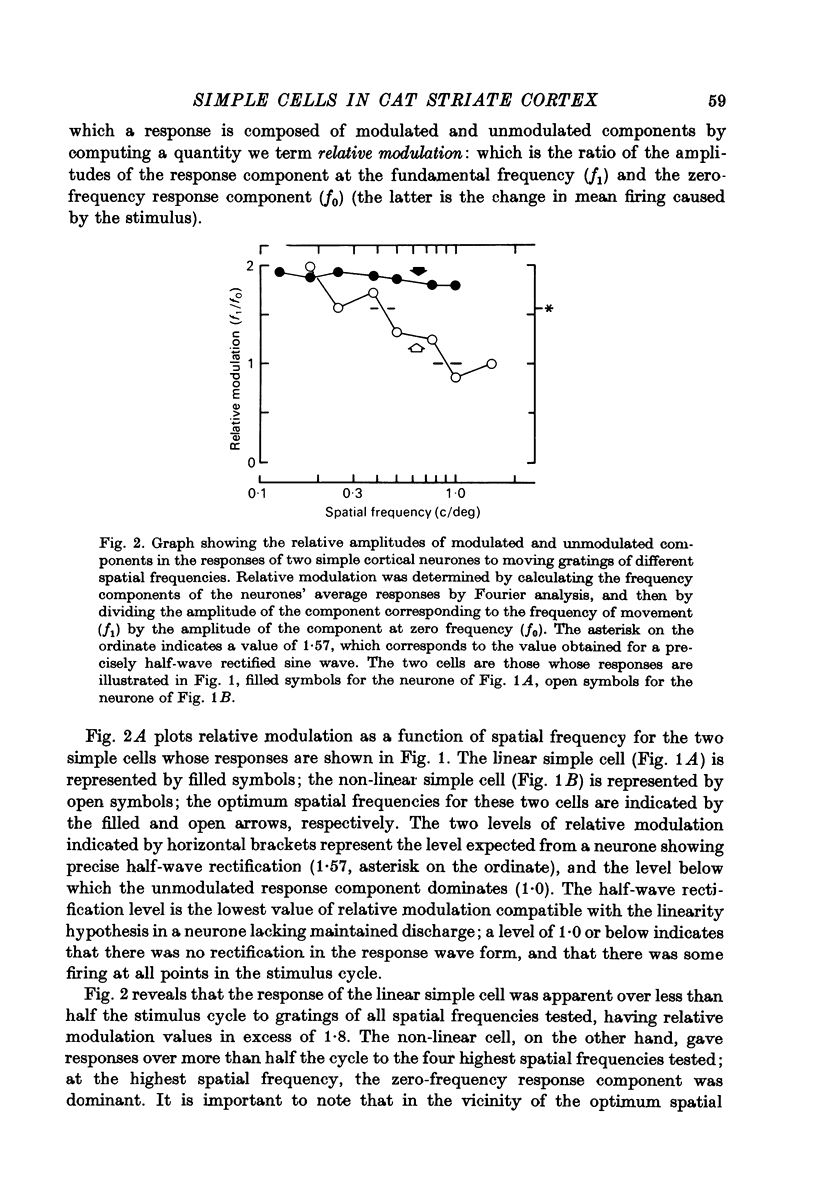
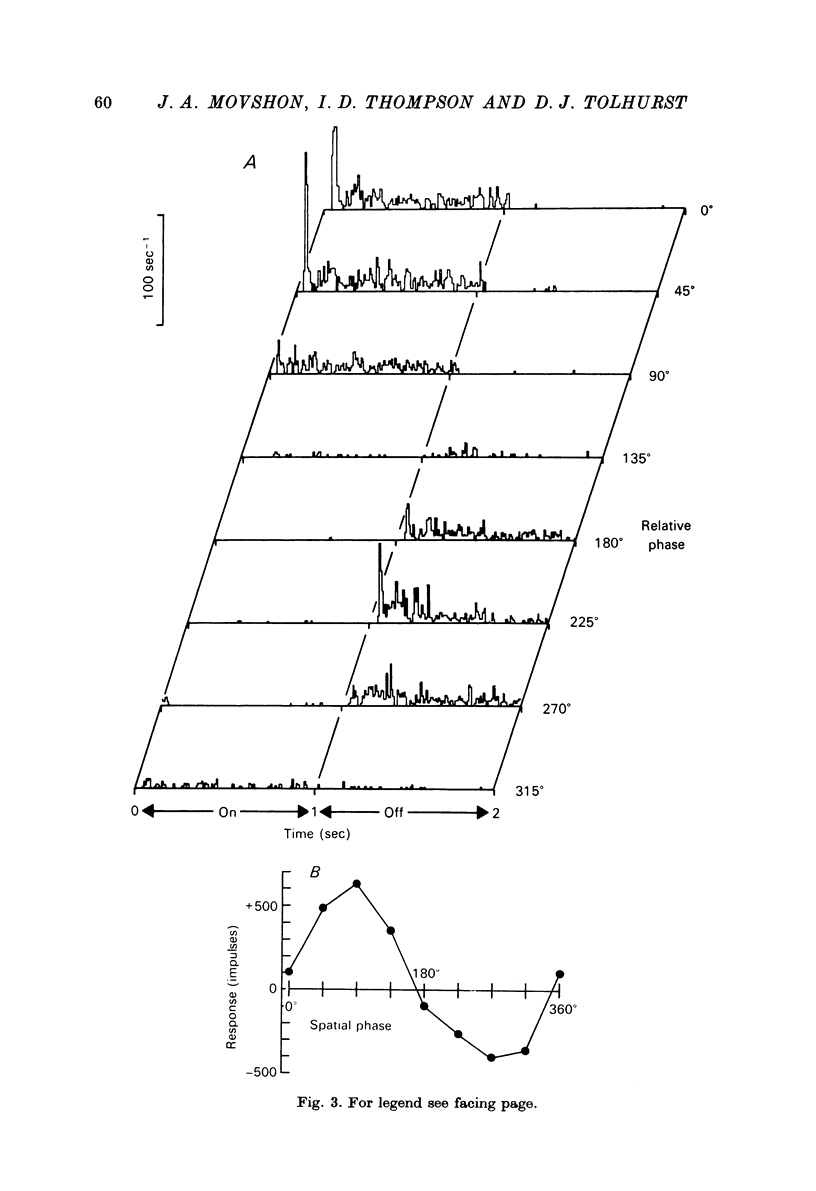
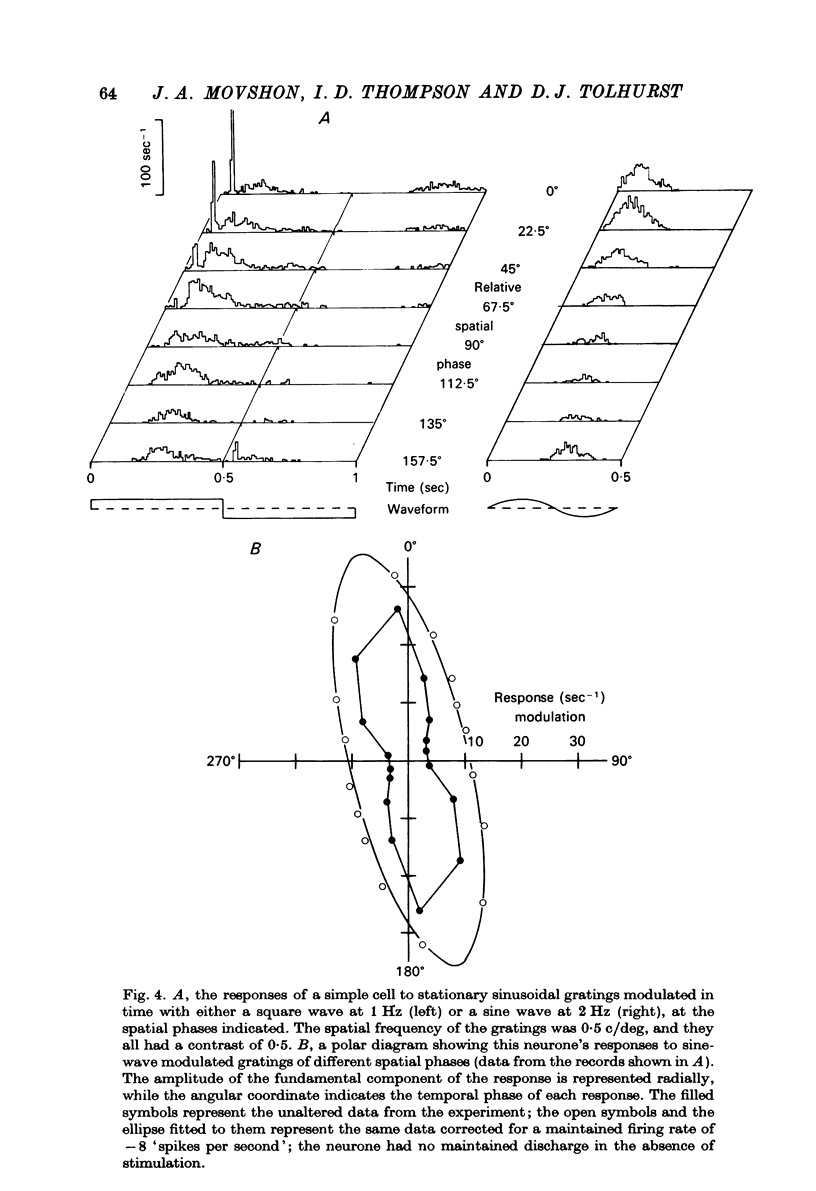
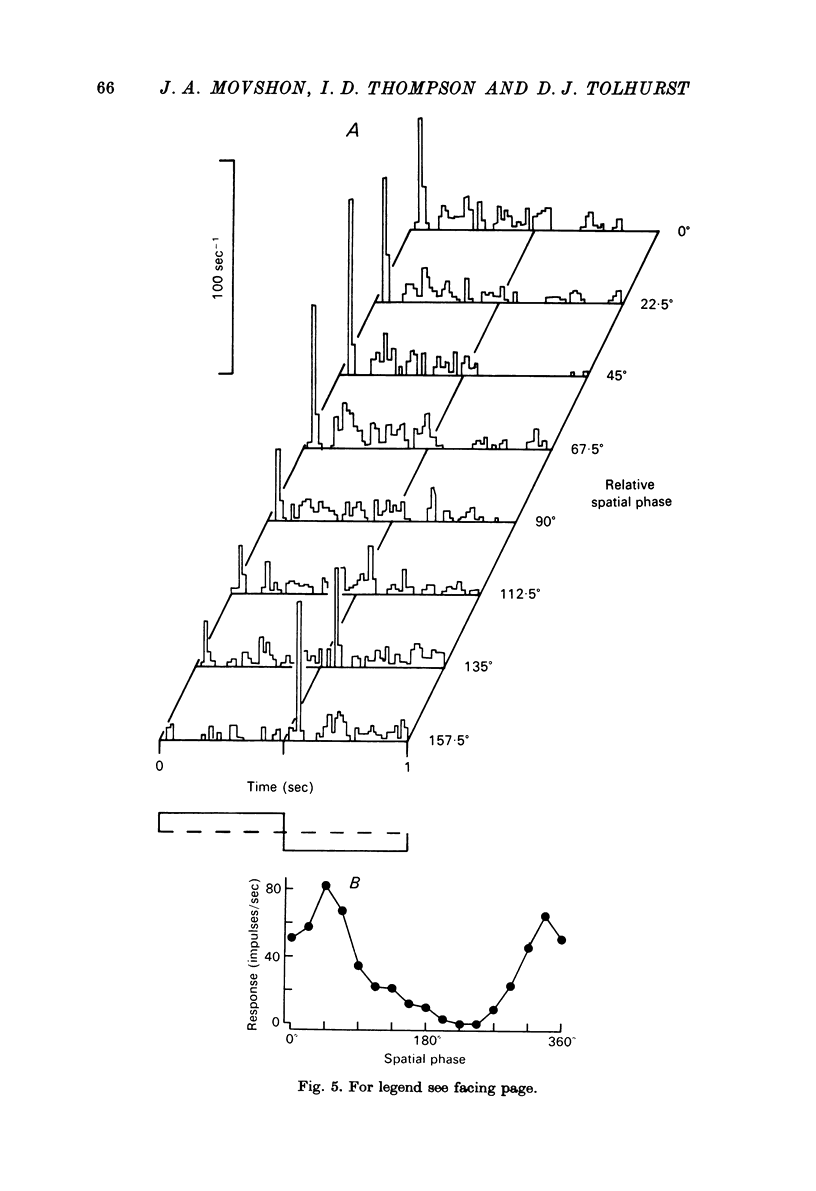
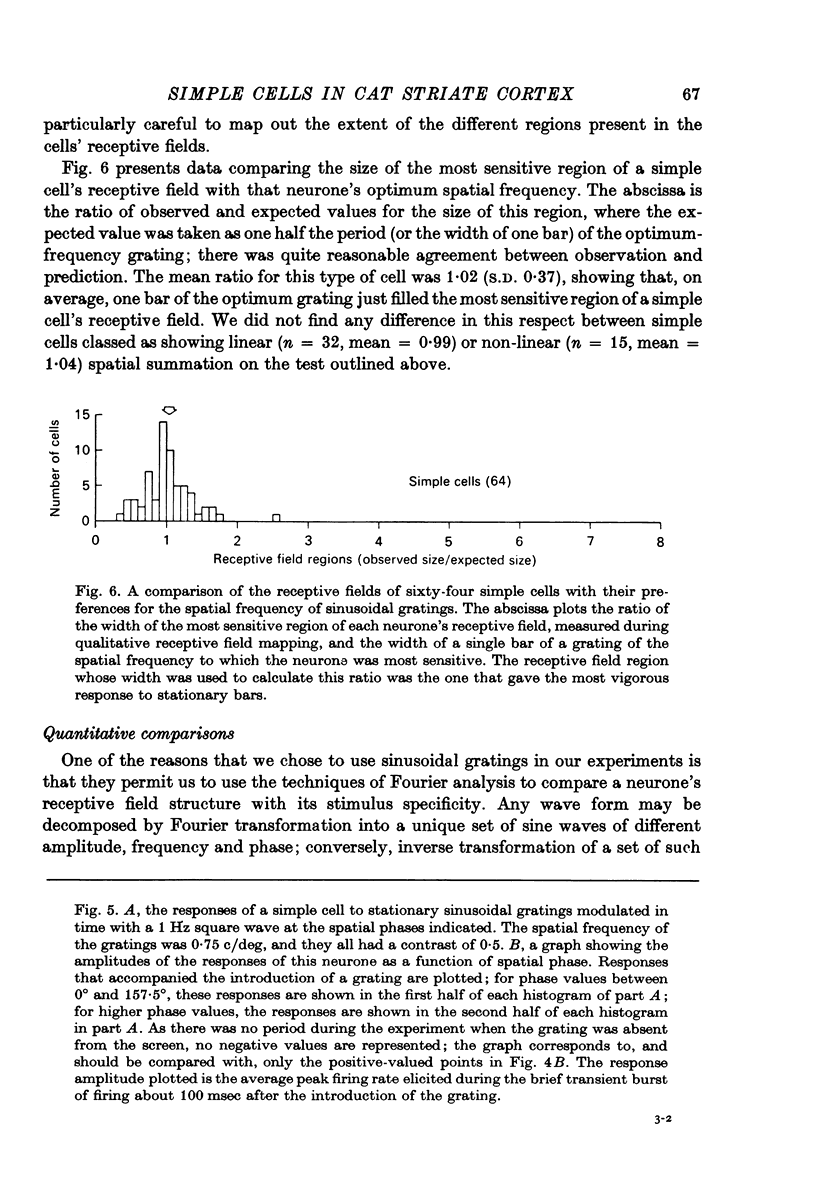
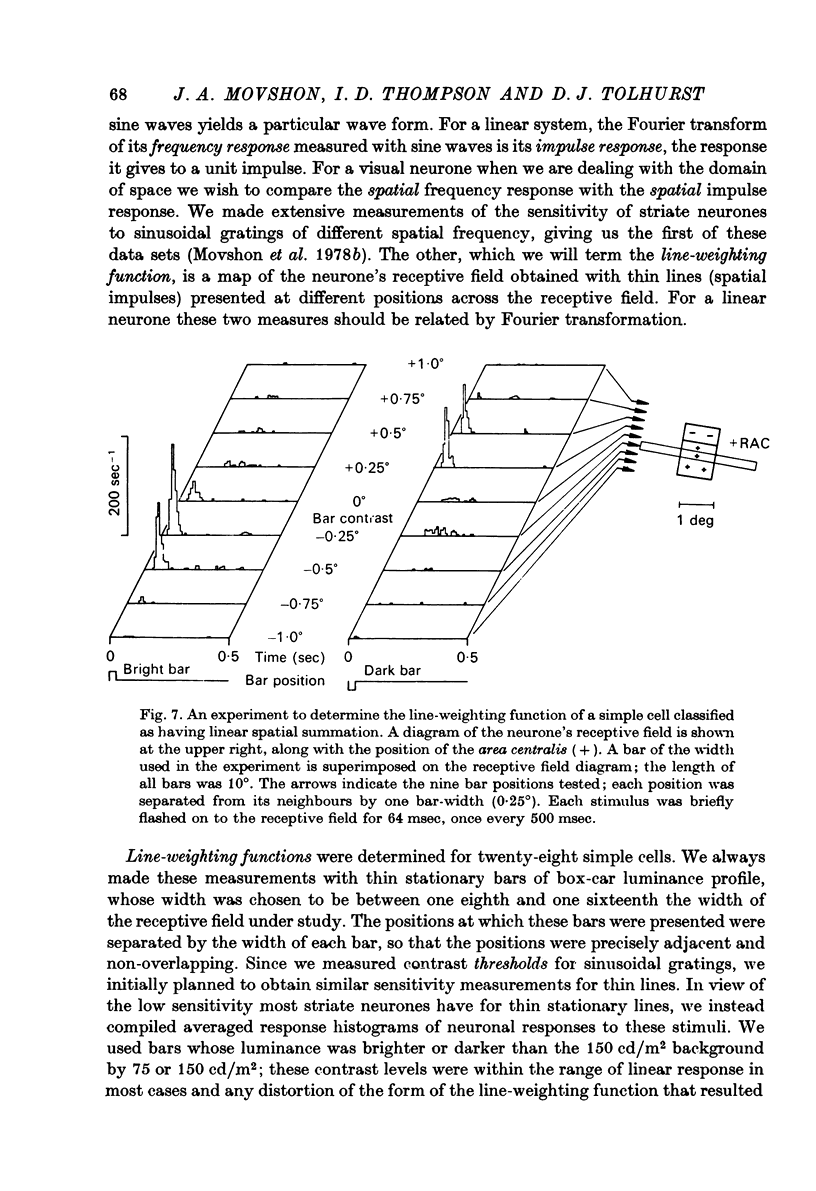
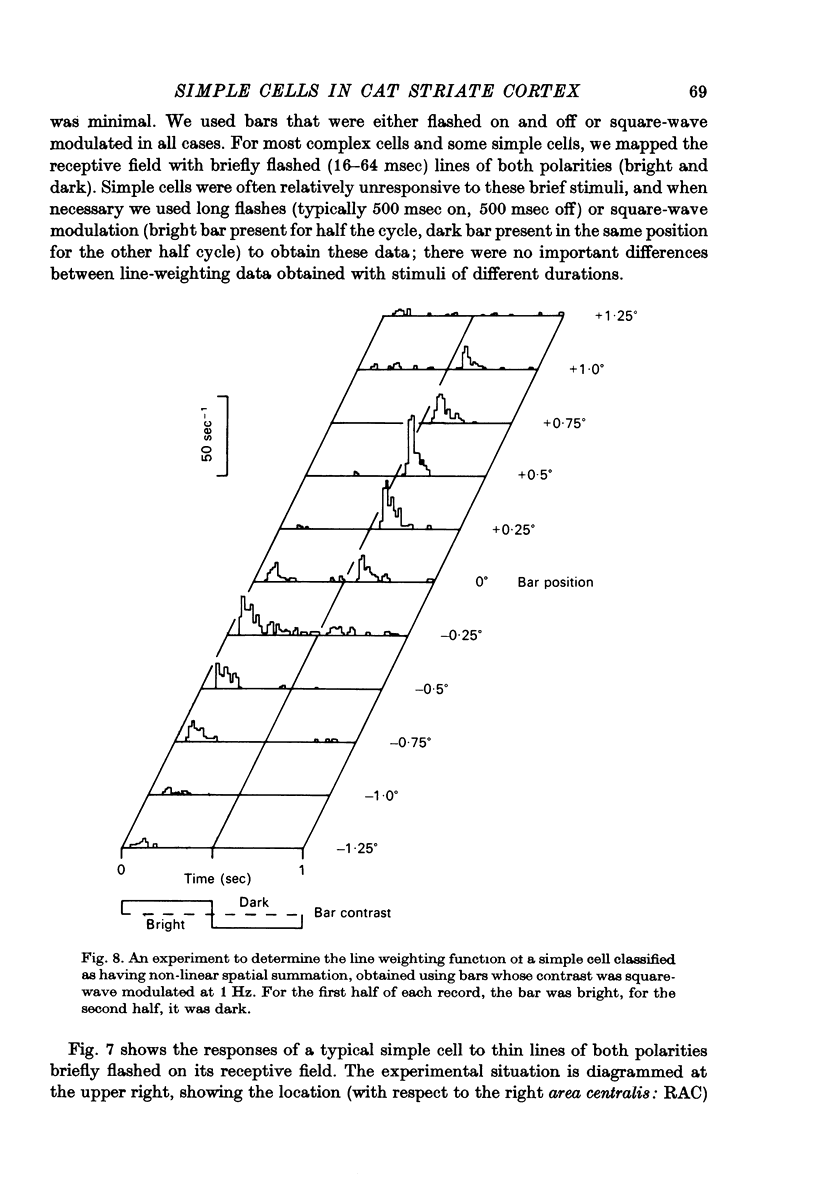
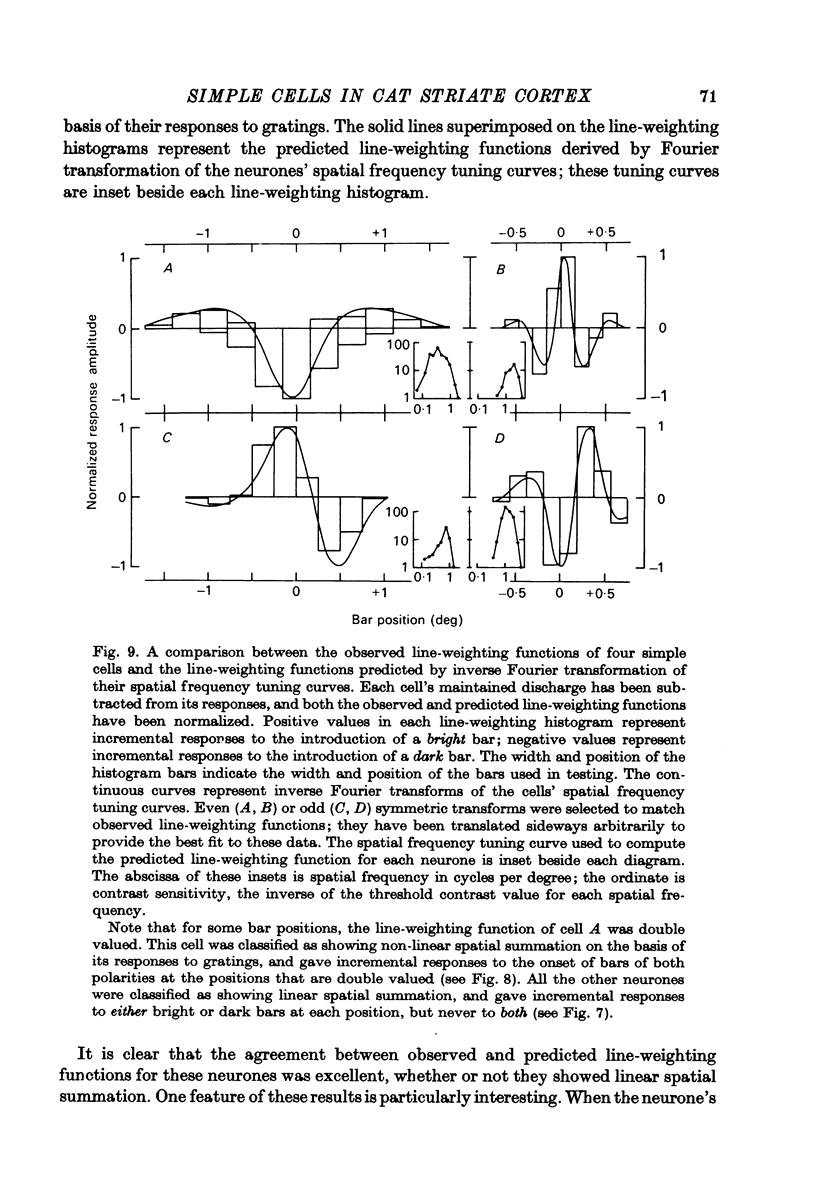
1. We have examined the responses of simple cells in the cat's atriate cortex to visual patterns that were designed to reveal the extent to which these cells may be considered to sum light-evoked influences linearly across their receptive fields. We used one-dimensional luminance-modulated bars and grating as stimuli; their orientation was always the same as the preferred orientation of the neurone under study. The stimuli were presented on an oscilloscope screen by a digital computer, which also accumulated neuronal responses and controlled a randomized sequence of stimulus presentations. 2. The majority of simple cells respond to sinusoidal gratings that are moving or whose contrast is modulated in time in a manner consistent with the hypothesis that they have linear spatial summation. Their responses to moving gratings of all spatial frequencies are modulated in synchrony with the passage of the gratings' bars across their receptive fields, and they do not produce unmodulated responses even at the highest spatial frequencies. Many of these cells respond to temporally modulated stationary gratings simply by changing their response amplitude sinusoidally as the spatial phase of the grating the grating is varied. Nonetheless, their behavior appears to indicate linear spatial summation, since we show in an Appendix that the absence of a 'null' phase in a visual neurone need not indicate non-linear spatial summation, and further that a linear neurone lacking a 'null' phase should give responses of the form that we have observed in this type of simple cell. 3. A minority of simple cells appears to have significant non-linearities of spatial summation. These neurones respond to moving gratings of high spatial frequency with a partially or totally unmodulated elevation of firing rate. They have no 'null' phases when tested with stationary gratings, and reveal their non-linearity by giving responses to gratings of some spatial phases that are composed partly or wholly of even harmonics of the stimulus frequency ('on-off' responses). 4. We compared simple receptive fields with their sensitivity to sinusoidal gratings of different spatial frequencies. Qualitatively, the most sensitive subregions of simple cells' receptive fields are roughly the same width as the individual bars of the gratings to which they are most sensitive. Quantitatively, their receptive field profiles measured with thin stationary lines, agree well with predicted profiles derived by Fourier synthesis of their spatial frequency tuning curves.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Interaction effects of visual contours on the discharge frequency of simple striate neurones. J Physiol. 1971 Dec;219(3):659–687. doi: 10.1113/jphysiol.1971.sp009682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Receptive fields of simple cells in the cat striate cortex. J Physiol. 1973 May;231(1):31–60. doi: 10.1113/jphysiol.1973.sp010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisti S., Maffei L. Behavioural contrast sensitivity of the cat in various visual meridians. J Physiol. 1974 Aug;241(1):201–210. doi: 10.1113/jphysiol.1974.sp010649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R., Cool S. J., Crawford M. L. Visual resolution in the cat. Vision Res. 1974 Nov;14(11):1211–1217. doi: 10.1016/0042-6989(74)90218-1. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Tobin E. A. Lateral inhibition between orientation detectors in the cat's visual cortex. Exp Brain Res. 1972;15(4):439–440. doi: 10.1007/BF00234129. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Cooper G. F., Enroth-Cugell C. The spatial selectivity of the visual cells of the cat. J Physiol. 1969 Jul;203(1):223–235. doi: 10.1113/jphysiol.1969.sp008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Kuhnt U., Benevento L. A. An intracellular analysis of visual cortical neurones to moving stimuli: response in a co-operative neuronal network. Exp Brain Res. 1974;21(3):251–274. doi: 10.1007/BF00235746. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSTEIN G. L., KIANG N. Y. An approach to the quantitative analysis of electrophysiological data from single neurons. Biophys J. 1960 Sep;1:15–28. doi: 10.1016/s0006-3495(60)86872-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin A. W., Henry G. H., Bishop P. O. Direction selectivity of simple striate cells: properties and mechanism. J Neurophysiol. 1975 Nov;38(6):1500–1523. doi: 10.1152/jn.1975.38.6.1500. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Dreher B. Orientation, axis and direction as stimulus parameters for striate cells. Vision Res. 1974 Sep;14(9):767–777. doi: 10.1016/0042-6989(74)90141-2. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Tupper R. M., Dreher B. Orientation specificity and response variability of cells in the striate cortex. Vision Res. 1973 Sep;13(9):1771–1779. doi: 10.1016/0042-6989(73)90094-1. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Dreher B., Bishop P. O. Orientation specificity of cells in cat striate cortex. J Neurophysiol. 1974 Nov;37(6):1394–1409. doi: 10.1152/jn.1974.37.6.1394. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973 Jul;13(7):1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Movshon J. A. Reversal of the physiological effects of monocular deprivation in the kitten's visual cortex. J Physiol. 1976 Sep;261(1):125–174. doi: 10.1113/jphysiol.1976.sp011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A. The velocity tuning of single units in cat striate cortex. J Physiol. 1975 Aug;249(3):445–468. doi: 10.1113/jphysiol.1975.sp011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Receptive field organization of complex cells in the cat's striate cortex. J Physiol. 1978 Oct;283:79–99. doi: 10.1113/jphysiol.1978.sp012489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J Physiol. 1978 Oct;283:101–120. doi: 10.1113/jphysiol.1978.sp012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Tolhurst D. J. Proceedings: The use of a digital computer in the study of neuronal properties in the visual system. J Physiol. 1976 Jan;254(1):2P–4P. [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Rose D., Blakemore C. An analysis of orientation selectivity in the cat's visual cortex. Exp Brain Res. 1974 Apr 30;20(1):1–17. doi: 10.1007/BF00239014. [DOI] [PubMed] [Google Scholar]
- SCHADE O. H., Sr Optical and photoelectric analog of the eye. J Opt Soc Am. 1956 Sep;46(9):721–739. doi: 10.1364/josa.46.000721. [DOI] [PubMed] [Google Scholar]
- Shapley R., Hochstein S. Visual spatial summation in two classes of geniculate cells. Nature. 1975 Jul 31;256(5516):411–413. doi: 10.1038/256411a0. [DOI] [PubMed] [Google Scholar]
- Tolhurst D. J., Movshon J. A. Spatial and temporal contrast sensitivity of striate cortical neurones. Nature. 1975 Oct 23;257(5528):674–675. doi: 10.1038/257674a0. [DOI] [PubMed] [Google Scholar]


