Abstract
1. All complex cells in the cat's striate cortex exhibit gross non-linearities of spatial summation when tested with sinusoidal grating stimuli. Their responses to moving gratings of all but the lowest spatial frequencies are usually dominated by a component that is not modulated by the passage of the bars of the grating across the receptive field. They give responses to temporally modulated stationary gratings that consist mostly of even harmonics of the stimulus frequency and that vary little in amplitude or wave form as the spatial phase of the grating is varied. 2. We compared complex cells' receptive fields with their sensitivity to sinusoidal gratings of different spatial frequencies. Qualitatively, the receptive fields are usually two to five times wider than the bars of the gratings that stimulate them most effectively. Quantitatively, the receptive field profiles of complex cells are invariably broader than those predicted by Fourier synthesis of their spatial frequency tuning curves, and in particular lack predicted spatially antagonistic regions. 3. We further examined the receptive field organization of these cells, using pairs of stationary lines flashed synchronously on their receptive fields. If both lines are of the same polarity (bright or dark), complex cells respond to the paired stimulus much less well than they do to either of its component bars, unless the bars are separated by less than about one quarter of the width of the receptive field. If the lines are of opposite polarity, one bright and one dark, the opposite situation obtains: closely spaced bars elicit small responses, while paired bars of larger separation are much more effective. In either case, the results are independent in general character of the absolute positions of the stimuli within the receptive field; rather, they depend in a manner characteristic of each cell on the relative positions of the two bars. 4. The two-line interaction profile that plots the change in a complex cell's response to one bar as a function of the position of a second added bar corresponds closely to the receptive field profile predicted from Fourier synthesis of the cell's spatial frequency tuning curve. These profiles may thus reveal the spatial characteristics of subunits within complex cell-receptive fields. We examined the nature of the interaction between these subunits by performing several two-line interaction experiments in which the onset of the second bar was delayed some time after the onset of the first. The results suggest that neighbouring subunits interact in a facilitatory fashion: for an interval after the presentation of one bar, responses to neighbouring bars are enhanced. 5. The subunits of a complex receptive field may, by their spatial properties, determine the spatial selectivities of complex cells, while the nature of the interaction among the subunits may determine these cells' sensitivity and selectivity for moving visual stimuli...
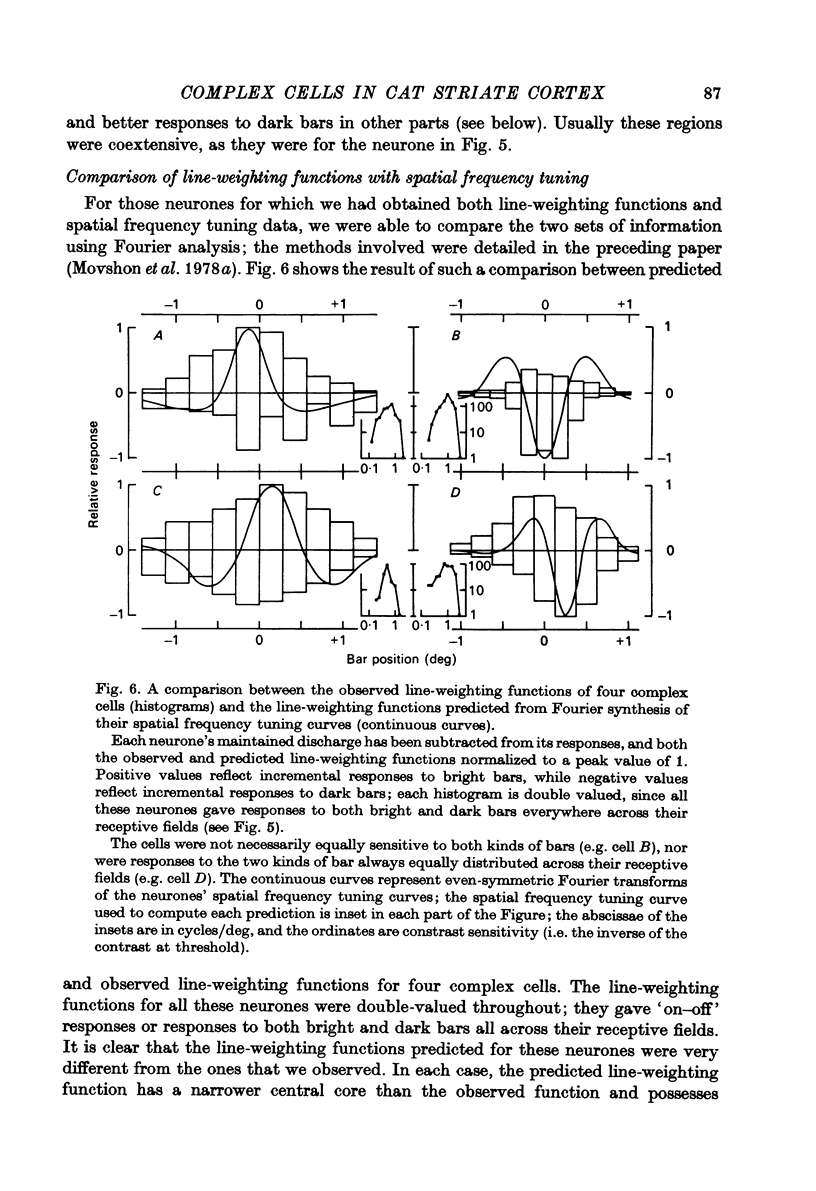
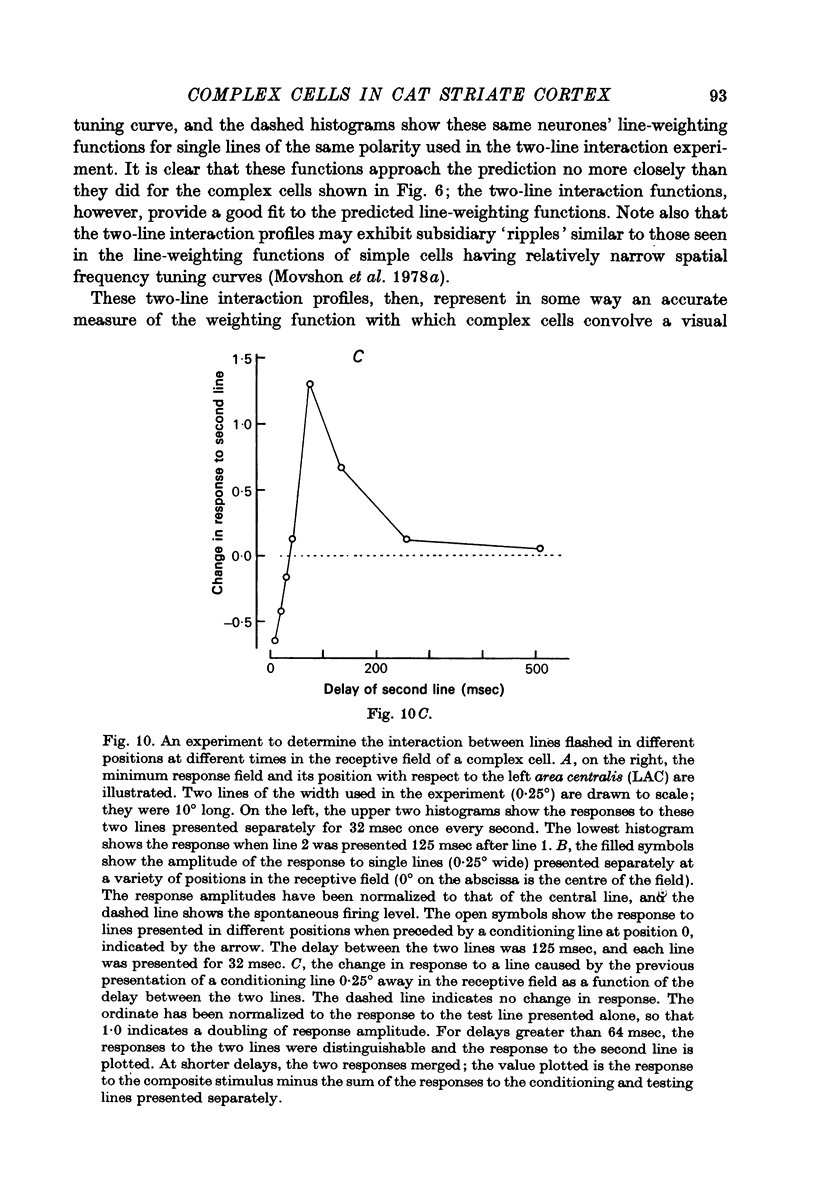
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965 Jun;178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M., Berman N., Hein A. Recovery of function in cat visual cortex following prolonged deprivation. Exp Brain Res. 1976 May 28;25(2):139–156. doi: 10.1007/BF00234899. [DOI] [PubMed] [Google Scholar]
- Daw N. W., Wyatt H. J. Kittens reared in a unidirectional environment: evidence for a critical period. J Physiol. 1976 May;257(1):155–170. doi: 10.1113/jphysiol.1976.sp011361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. L., Schiller P. H., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. IV. Corticotectal cells. J Neurophysiol. 1976 Nov;39(6):1352–1361. doi: 10.1152/jn.1976.39.6.1352. [DOI] [PubMed] [Google Scholar]
- Foster D. H. A model of the human visual system in its response to certain classes of moving stimuli. Kybernetik. 1971 Feb;8(2):69–84. doi: 10.1007/BF00288735. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin A. W., Henry G. H., Bishop P. O. Direction selectivity of simple striate cells: properties and mechanism. J Neurophysiol. 1975 Nov;38(6):1500–1523. doi: 10.1152/jn.1975.38.6.1500. [DOI] [PubMed] [Google Scholar]
- Goodwin A. W., Henry G. H. Direction selectivity of complex cells in a comparison with simple cells. J Neurophysiol. 1975 Nov;38(6):1524–1540. doi: 10.1152/jn.1975.38.6.1524. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Dreher B. Orientation, axis and direction as stimulus parameters for striate cells. Vision Res. 1974 Sep;14(9):767–777. doi: 10.1016/0042-6989(74)90141-2. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua D. E., Bishop P. O. Binocular single vision and depth discrimination. Receptive field disparities for central and peripheral vision and binocular interaction on peripheral single units in cat striate cortex. Exp Brain Res. 1970;10(4):389–416. doi: 10.1007/BF02324766. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J Physiol. 1978 Oct;283:101–120. doi: 10.1113/jphysiol.1978.sp012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J Physiol. 1978 Oct;283:53–77. doi: 10.1113/jphysiol.1978.sp012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Binocular interaction on single units in cat striate cortex: simultaneous stimulation by single moving slit with receptive fields in correspondence. Exp Brain Res. 1968;6(4):391–410. doi: 10.1007/BF00233186. [DOI] [PubMed] [Google Scholar]
- Poggio T., Reichardt W. Visual control of orientation behaviour in the fly. Part II. Towards the underlying neural interactions. Q Rev Biophys. 1976 Aug;9(3):377–438. doi: 10.1017/s0033583500002535. [DOI] [PubMed] [Google Scholar]
- Rose D. Responses of single units in cat visual cortex to moving bars of light as a function of bar length. J Physiol. 1977 Sep;271(1):1–23. doi: 10.1113/jphysiol.1977.sp011987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlag-Rey M., Schlag J. Visual and presaccadic neuronal activity in thalamic internal medullary lamina of cat: a study of targeting. J Neurophysiol. 1977 Jan;40(1):156–173. doi: 10.1152/jn.1977.40.1.156. [DOI] [PubMed] [Google Scholar]
- Shapley R., Hochstein S. Visual spatial summation in two classes of geniculate cells. Nature. 1975 Jul 31;256(5516):411–413. doi: 10.1038/256411a0. [DOI] [PubMed] [Google Scholar]
- Wyatt H. J., Daw N. W. Directionally sensitive ganglion cells in the rabbit retina: specificity for stimulus direction, size, and speed. J Neurophysiol. 1975 May;38(3):613–626. doi: 10.1152/jn.1975.38.3.613. [DOI] [PubMed] [Google Scholar]


