Abstract
Striated muscles from Rana pipiens have been exposed for variable periods of time to Ringer solutions made hypertonic by addition of either sucrose or sodium chloride. The muscles have been rapid-frozen and then prepared for electron microscopy by either freeze-substitution, freeze-fracture or cryoultramicrotomy. The only compartment greatly affected by hypertonicity is the transverse tubular system, which is visibly swollen. None of the elements of the sarcoplasmic reticulum increase in size.
Full text
PDF







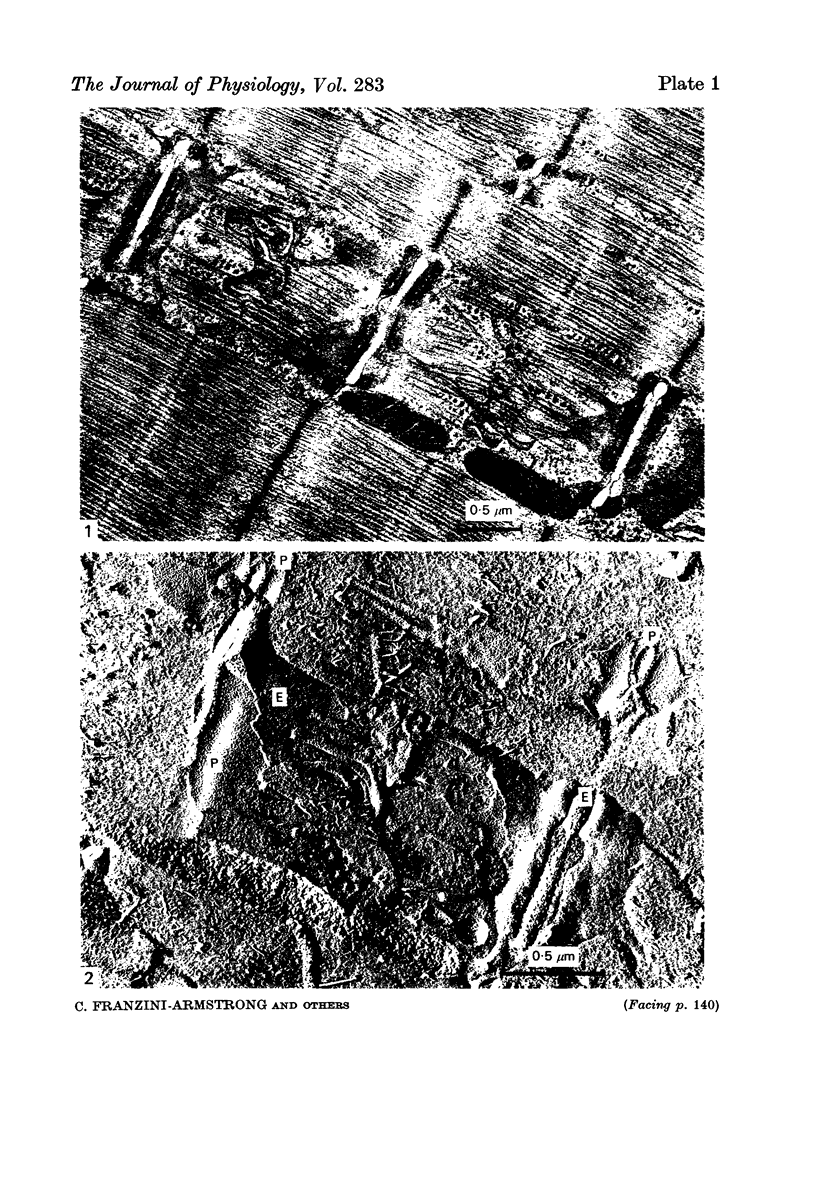

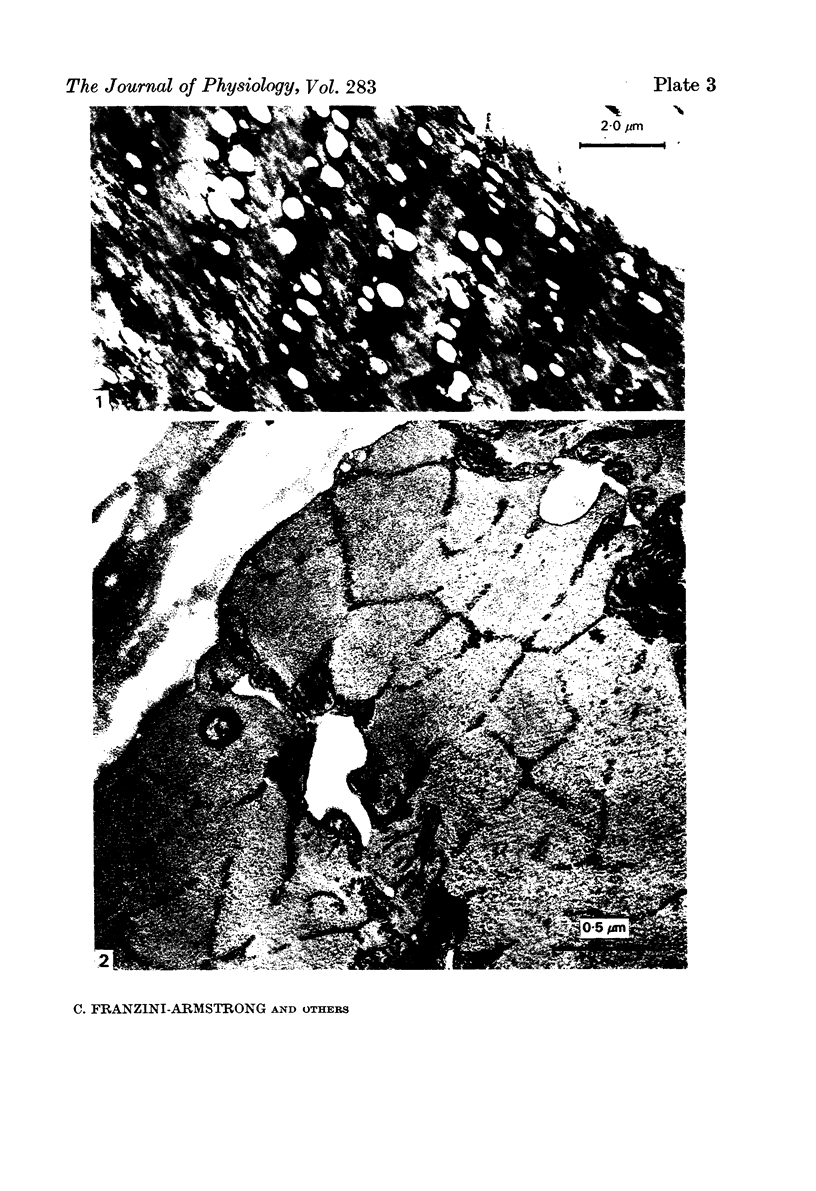

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton T. C. A cryostat approach to ultrathin "dry" frozen sections for electron microscopy: a morphological and x-ray analytical study. J Microsc. 1974 Jan;100(1):49–74. doi: 10.1111/j.1365-2818.1974.tb03913.x. [DOI] [PubMed] [Google Scholar]
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian J., Nakajima S. Action potential in the transverse tubules and its role in the activation of skeletal muscle. J Gen Physiol. 1974 Feb;63(2):257–278. doi: 10.1085/jgp.63.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Davey D. F. An analysis of volume changes in the T-tubes of frog skeletal muscle exposed to sucrose. J Physiol. 1972 Apr;222(1):95–111. doi: 10.1113/jphysiol.1972.sp009789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Davey D. F. Osmotic responses demonstrating the extracellular character of the sarcoplasmic reticulum. J Physiol. 1969 May;202(1):171–188. doi: 10.1113/jphysiol.1969.sp008802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY E. J. Nature and significance of concentration relations of potassium and sodium ions in skeletal muscle. Physiol Rev. 1957 Jan;37(1):84–132. doi: 10.1152/physrev.1957.37.1.84. [DOI] [PubMed] [Google Scholar]
- Christensen A. K. Frozen thin sections of fresh tissue for electron microscopy, with a description of pancreas and liver. J Cell Biol. 1971 Dec;51(3):772–804. doi: 10.1083/jcb.51.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantin L. L. Contractile activation in skeletal muscle. Prog Biophys Mol Biol. 1975;29(2):197–224. doi: 10.1016/0079-6107(76)90023-7. [DOI] [PubMed] [Google Scholar]
- Costantin L. L. The role of sodium current in the radial spread of contraction in frog muscle fibers. J Gen Physiol. 1970 Jun;55(6):703–715. doi: 10.1085/jgp.55.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYDYNSKA M., WILKIE D. R. THE OSMOTIC PROPERTIES OF STRIATED MUSCLE FIBERS IN HYPERTONIC SOLUTIONS. J Physiol. 1963 Nov;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYDYNSKA M., WILKIE D. R. THE OSMOTIC PROPERTIES OF STRIATED MUSCLE FIBERS IN HYPERTONIC SOLUTIONS. J Physiol. 1963 Nov;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERANKO O. Quenching of tissues for freeze-drying. Acta Anat (Basel) 1954;22(4):331–336. [PubMed] [Google Scholar]
- FALK G., FATT P. LINEAR ELECTRICAL PROPERTIES OF STRIATED MUSCLE FIBRES OBSERVED WITH INTRACELLULAR ELECTRODES. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:69–123. doi: 10.1098/rspb.1964.0030. [DOI] [PubMed] [Google Scholar]
- FEDER N., SIDMAN R. L. Methods and principles of fixation by freeze-substitution. J Biophys Biochem Cytol. 1958 Sep 25;4(5):593–600. doi: 10.1083/jcb.4.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANZINI-ARMSTRONG C., PORTER K. R. SARCOLEMMAL INVAGINATIONS CONSTITUTING THE T SYSTEM IN FISH MUSCLE FIBERS. J Cell Biol. 1964 Sep;22:675–696. doi: 10.1083/jcb.22.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes M. S., Plantholt B. A., Sperelakis N. Cytochemical staining procedures selective for sarcotubular systems of muscle: modifications and applications. J Ultrastruct Res. 1977 Sep;60(3):306–327. doi: 10.1016/s0022-5320(77)80016-6. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. STUDIES OF THE TRIAD : I. Structure of the Junction in Frog Twitch Fibers. J Cell Biol. 1970 Nov 1;47(2):488–499. doi: 10.1083/jcb.47.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freygang W. H., Jr, Rapoport S. I., Peachey L. D. Some relations between changes in the linear electrical properties of striated muscle fibers and changes in ultrastructure. J Gen Physiol. 1967 Nov;50(10):2437–2458. doi: 10.1085/jgp.50.10.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Serratos H. Inward spread of activation in vertebrate muscle fibres. J Physiol. 1971 Feb;212(3):777–799. doi: 10.1113/jphysiol.1971.sp009356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J. Distribution and movement of muscle chloride. J Physiol. 1963 Apr;166:87–109. doi: 10.1113/jphysiol.1963.sp007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. EVIDENCE FOR CONTINUITY BETWEEN THE CENTRAL ELEMENTS OF THE TRIADS AND EXTRACELLULAR SPACE IN FROG SARTORIUS MUSCLE. Nature. 1964 Jun 13;202:1067–1071. doi: 10.1038/2021067b0. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Preservation of synaptic structure by rapid freezing. Cold Spring Harb Symp Quant Biol. 1976;40:17–24. doi: 10.1101/sqb.1976.040.01.004. [DOI] [PubMed] [Google Scholar]
- Howell J. N. Intracellular binding of ruthenium red in frog skeletal muscle. J Cell Biol. 1974 Jul;62(1):242–247. doi: 10.1083/jcb.62.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Steinhardt R. A. The components of the sodium efflux in frog muscle. J Physiol. 1968 Oct;198(3):581–599. doi: 10.1113/jphysiol.1968.sp008627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- PORTER K. R., PALADE G. E. Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. J Biophys Biochem Cytol. 1957 Mar 25;3(2):269–300. doi: 10.1083/jcb.3.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E., Upshaw-Earley J. Volume changes in sarcoplasmic reticulum of rat hearts perfused with hypertonic solutions. Circ Res. 1977 Apr;40(4):355–366. doi: 10.1161/01.res.40.4.355. [DOI] [PubMed] [Google Scholar]
- Rogus E., Zierler K. L. Sodium and water contents of sarcoplasm and sarcoplasmic reticulum in rat skeletal muscle: effects of anisotonic media, ouabain and external sodium. J Physiol. 1973 Sep;233(2):227–270. doi: 10.1113/jphysiol.1973.sp010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome E. X-ray diffraction studies of the filament lattice of striated muscle in various bathing media. J Mol Biol. 1968 Oct 28;37(2):331–344. doi: 10.1016/0022-2836(68)90272-6. [DOI] [PubMed] [Google Scholar]
- Rubio R., Sperelakis N. Penetration of horseradish peroxidase into the terminal cisternae of frog skeletal muscle fibers and blockade of caffeine contracture by Ca ++ depletion. Z Zellforsch Mikrosk Anat. 1972;124(1):57–71. [PubMed] [Google Scholar]
- Smith D. S. THE STRUCTURE OF INSECT FIBRILLAR FLIGHT MUSCLE : A Study Made with Special Reference to the Membrane Systems of the Fiber. J Biophys Biochem Cytol. 1961 Aug 1;10(4):123–158. doi: 10.1083/jcb.10.4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Shuman H., Somlyo A. P. Composition of sarcoplasmic reticulum in situ by electron probe X-ray microanalysis. Nature. 1977 Aug 11;268(5620):556–558. doi: 10.1038/268556a0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Shuman H., Somlyo A. P. Elemental distribution in striated muscle and the effects of hypertonicity. Electron probe analysis of cryo sections. J Cell Biol. 1977 Sep;74(3):828–857. doi: 10.1083/jcb.74.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHARREVELD A., CROWELL J. ELECTRON MICROSCOPY AFTER RAPID FREEZING ON A METAL SURFACE AND SUBSTITUTION FIXATION. Anat Rec. 1964 Jul;149:381–385. doi: 10.1002/ar.1091490307. [DOI] [PubMed] [Google Scholar]
- VANHARREVELD A., CROWELL J., MALHOTRA S. K. A STUDY OF EXTRACELLULAR SPACE IN CENTRAL NERVOUS TISSUE BY FREEZE-SUBSTITUTION. J Cell Biol. 1965 Apr;25:117–137. doi: 10.1083/jcb.25.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]









