Abstract
1. Voltage clamp experiments using the three micro-electrode voltage clamp technique were performed on sartorius muscles of the frog. 2. By blocking potassium currents with tetraethylammonium and replacing chloride ions with sulphate a slow inward current was detected. 3. The slow inward current is mainly carried by calcium, since it is abolished by cobalt and D-600, it depends on external calcium, and is not affected by removing external sodium or by tetrodotoxin (TTX). 4. The slow inward current has a mean threshold of -40 mV, reaches a mean maximum value at ca. 0 mV of 81 microamperemetercm-2 and has a mean reversal potential of +38 mV. 5. The calcium current is inactivated by the application of 2 sec conditioning prepulses according to a sigmoid curve with V(h) = -42 mV and k = 6.2 mV. 6. The slow time course of this calcium current makes it rather unlikely that it participates in contraction during a twitch, but it might be activated during long depolarizations as potassium contractures.
Full text
PDF


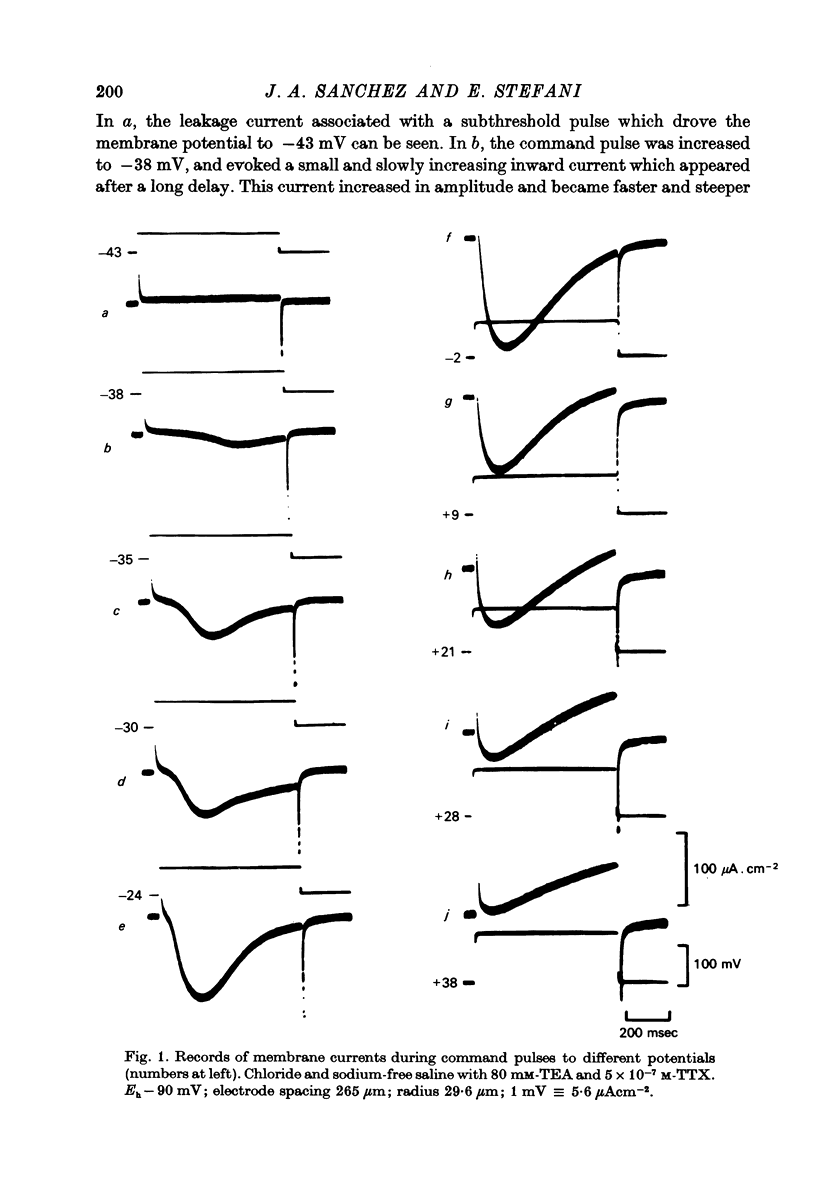

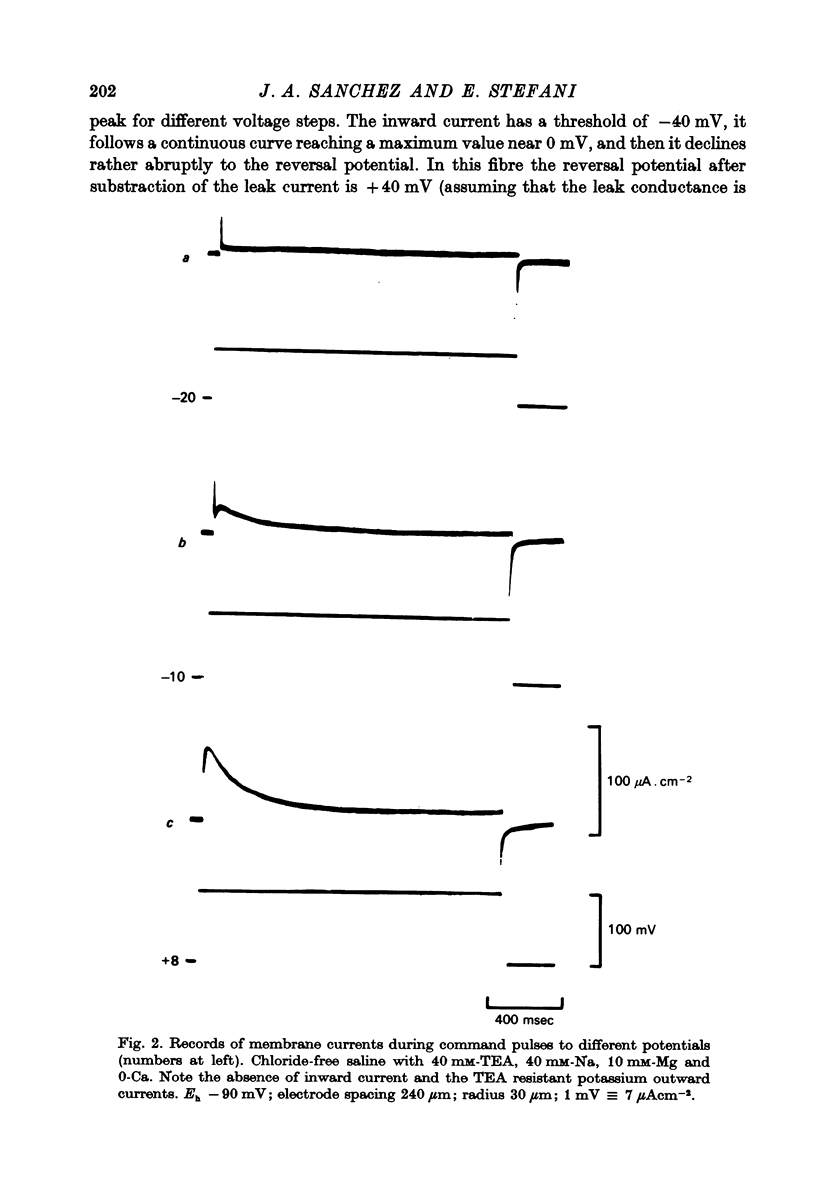
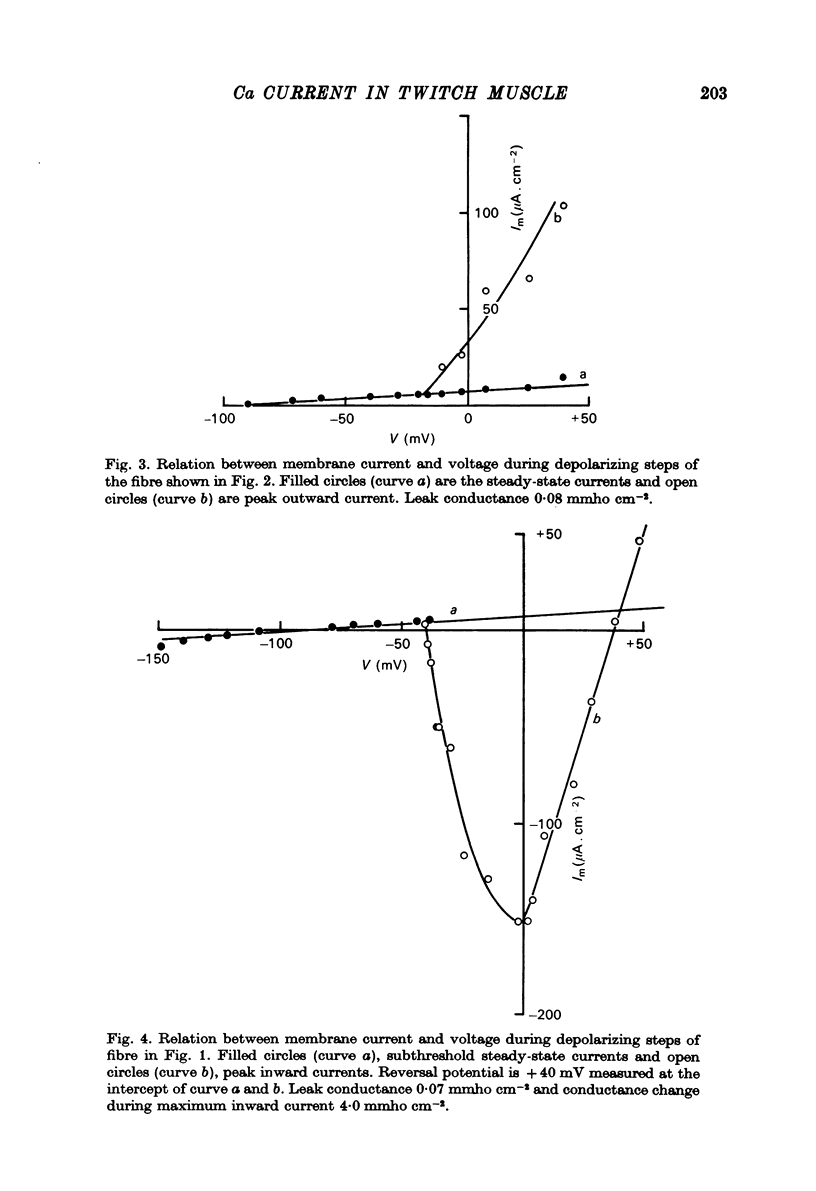
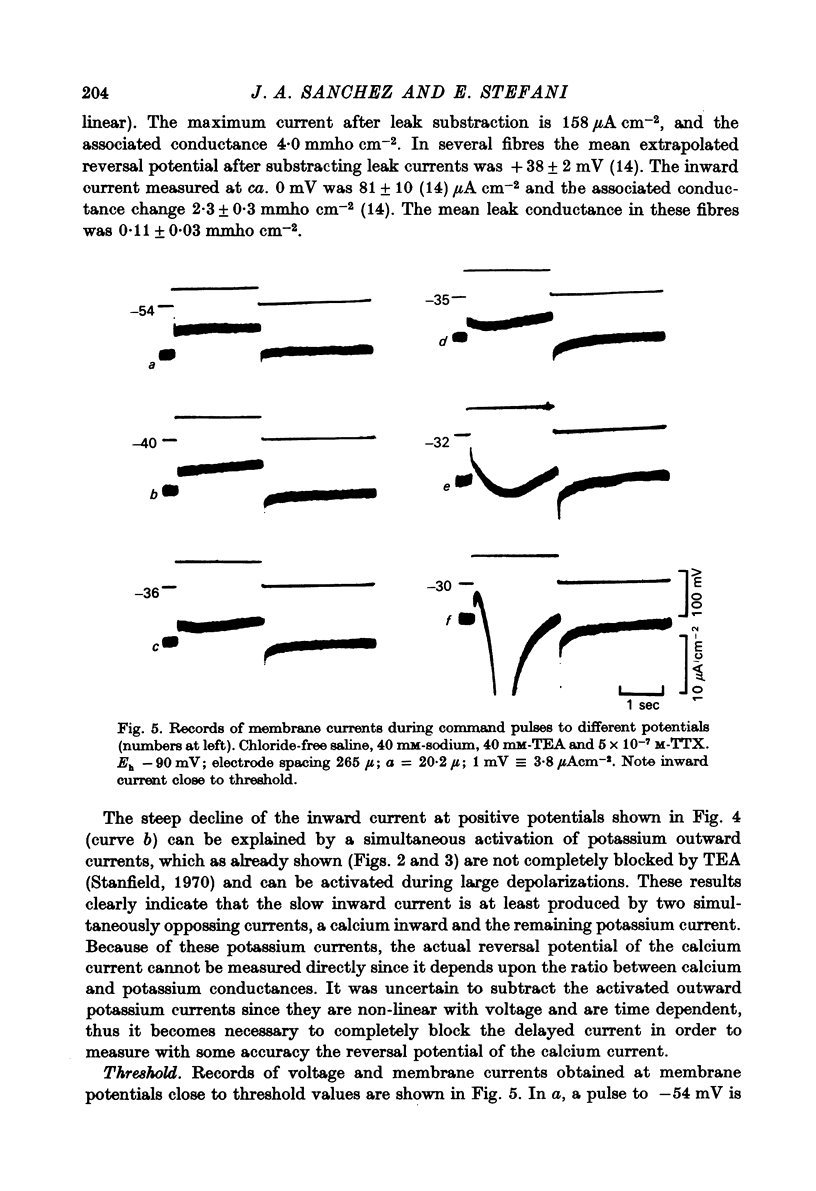
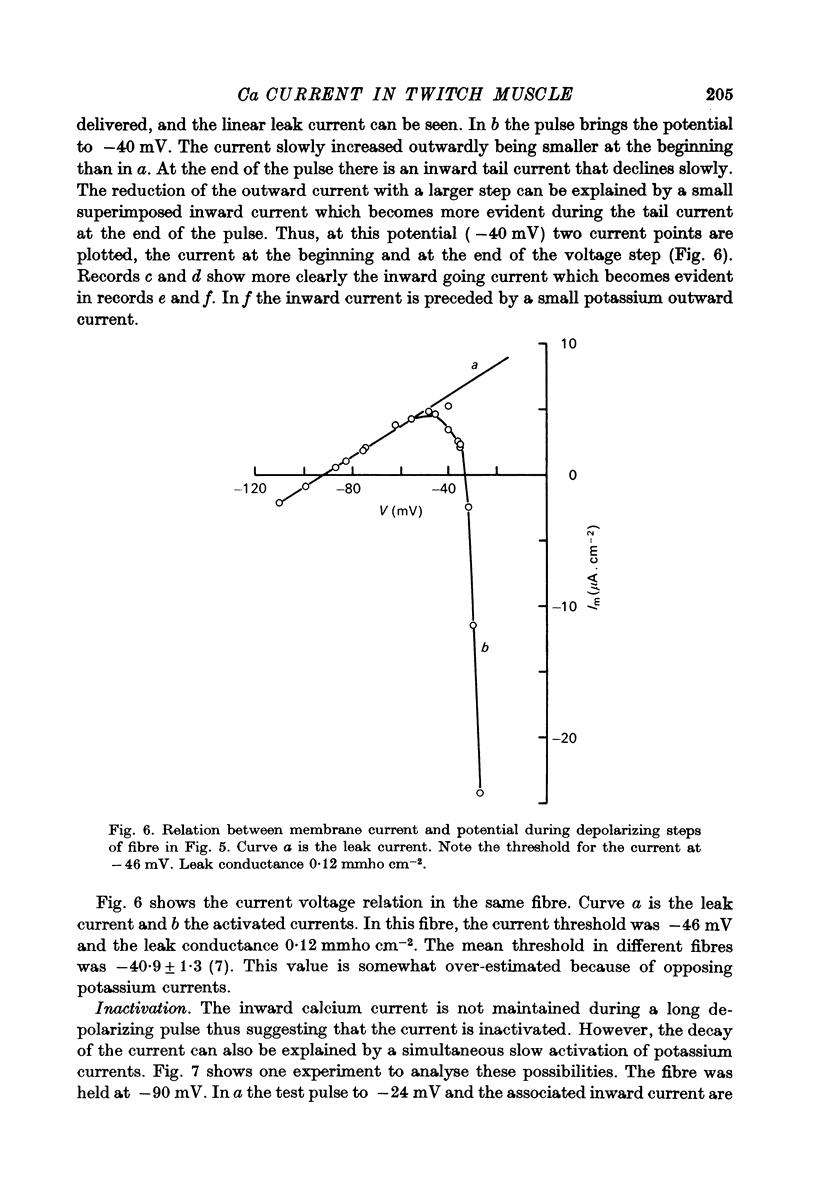



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. M., Horowicz P. Twitches in the presence of ethylene glycol bis( -aminoethyl ether)-N,N'-tetracetic acid. Biochim Biophys Acta. 1972 Jun 23;267(3):605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
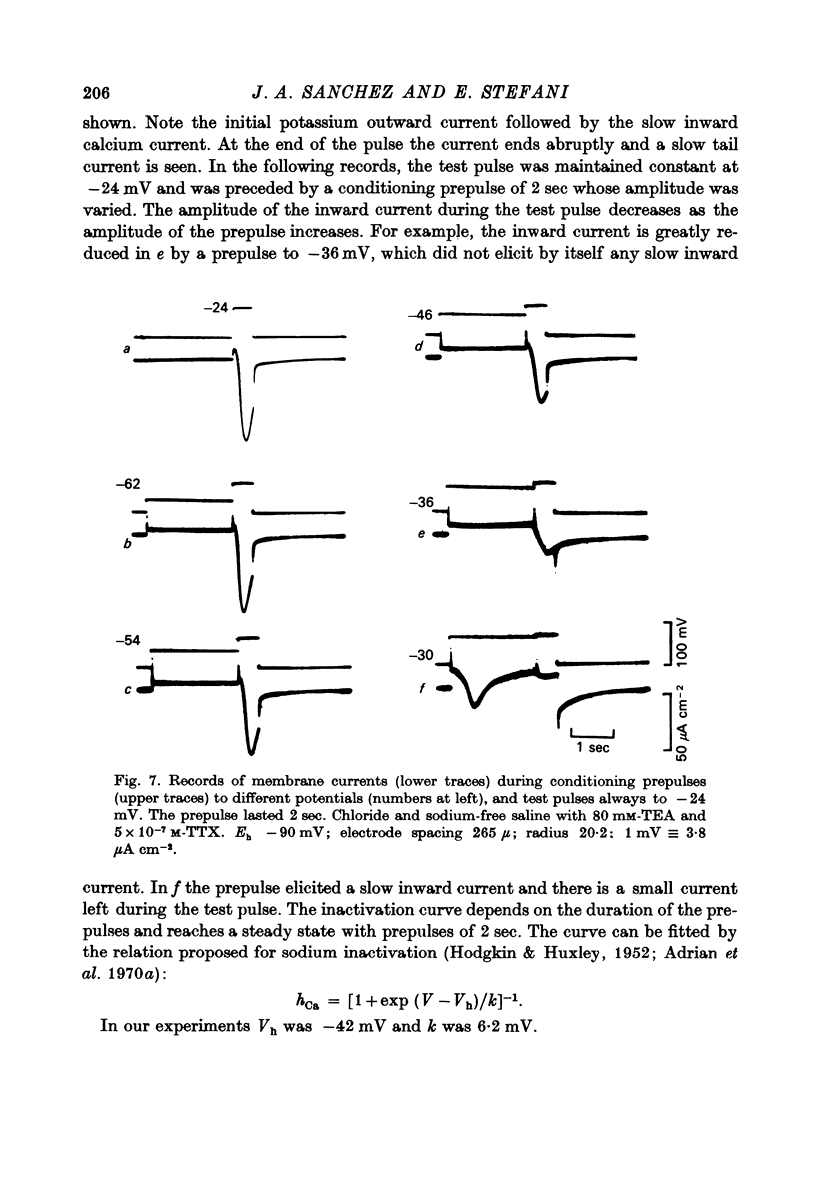
- BIANCHI C. P., SHANES A. M. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol. 1959 Mar 20;42(4):803–815. doi: 10.1085/jgp.42.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty G. N., Stefani E. Calcium dependent electrical activity in twitch muscle fibres of the frog. Proc R Soc Lond B Biol Sci. 1976 Aug 27;194(1114):141–150. doi: 10.1098/rspb.1976.0070. [DOI] [PubMed] [Google Scholar]
- Chiarandini D. J., Stefani E. Effects of manganese on the electrical and mechanical properties of frog skeletal muscle fibres. J Physiol. 1973 Jul;232(1):129–147. doi: 10.1113/jphysiol.1973.sp010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis B. A. Ca fluxes in single twitch muscle fibers. J Gen Physiol. 1966 Nov;50(2):255–267. doi: 10.1085/jgp.50.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis K. O., Carpenter J. F. Studies on the mechanism of action of dantrolene sodium. A skeletal muscle relaxant. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(1):83–94. doi: 10.1007/BF00505069. [DOI] [PubMed] [Google Scholar]
- Fink R., Lüttgau H. C. An evaluation of the membrane constants and the potassium conductance in metabolically exhausted muscle fibres. J Physiol. 1976 Dec;263(2):215–238. doi: 10.1113/jphysiol.1976.sp011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Movements of Na and K in single muscle fibres. J Physiol. 1959 Mar 3;145(2):405–432. doi: 10.1113/jphysiol.1959.sp006150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARTH J. V. The behaviour of frog muscle in hypertonic solutions. J Physiol. 1958 Nov 10;144(1):167–175. doi: 10.1113/jphysiol.1958.sp006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Hayashi H., Takahashi K. Calcium and potassium currents of the membrane of a barnacle muscle fibre in relation to the calcium spike. J Physiol. 1969 Nov;205(1):115–129. doi: 10.1113/jphysiol.1969.sp008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Pharmacological modifications of the sodium channels of frog nerve. J Gen Physiol. 1968 Feb;51(2):199–219. doi: 10.1085/jgp.51.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E., Taylor R. E., Vergara J. Calcium and potassium systems of a giant barnacle muscle fibre under membrane potential control. J Physiol. 1973 Mar;229(2):409–455. doi: 10.1113/jphysiol.1973.sp010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Selective inhibition of the transmembrane Ca conductivity of mammalian myocardial fibres by Ni, Co and Mn ions. Pflugers Arch. 1973 Jan 22;338(2):115–123. doi: 10.1007/BF00592747. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Two components of the calcium current in the egg cell membrane of the tunicate. J Physiol. 1976 Feb;255(2):527–561. doi: 10.1113/jphysiol.1976.sp011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Biancri C. P. Site of action of dantrolene in frog sartorius muscle. J Pharmacol Exp Ther. 1974 Apr;189(1):202–212. [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Slow inactivation of currents in cardiac Purkinje fibres. J Physiol. 1968 Jul;197(1):233–253. doi: 10.1113/jphysiol.1968.sp008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. A calcium dependent inward current in frog skeletal muscle fibres. Pflugers Arch. 1977 Apr 25;368(3):267–270. doi: 10.1007/BF00585206. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Chiarandini D. J. Skeletal muscle: dependence of potassium contractures on extracellular calcium. Pflugers Arch. 1973 Oct 17;343(2):143–150. doi: 10.1007/BF00585709. [DOI] [PubMed] [Google Scholar]
- Stefani E., Schmidt H. A convenient method for repeated intracellular recording of action potentials from the same muscle fibre without membrane damage. Pflugers Arch. 1972;334(3):276–278. doi: 10.1007/BF00626229. [DOI] [PubMed] [Google Scholar]
- Stefani E., Uchitel O. D. Potassium and calcium conductance in slow muscle fibres of the toad. J Physiol. 1976 Feb;255(2):435–448. doi: 10.1113/jphysiol.1976.sp011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassort G., Rougier O., Garnier D., Sauviat M. P., Coraboeuf E., Gargouïl Y. M. Effects of adrenaline on membrane inward currents during the cardiac action potential. Pflugers Arch. 1969;309(1):70–81. doi: 10.1007/BF00592283. [DOI] [PubMed] [Google Scholar]


