Abstract
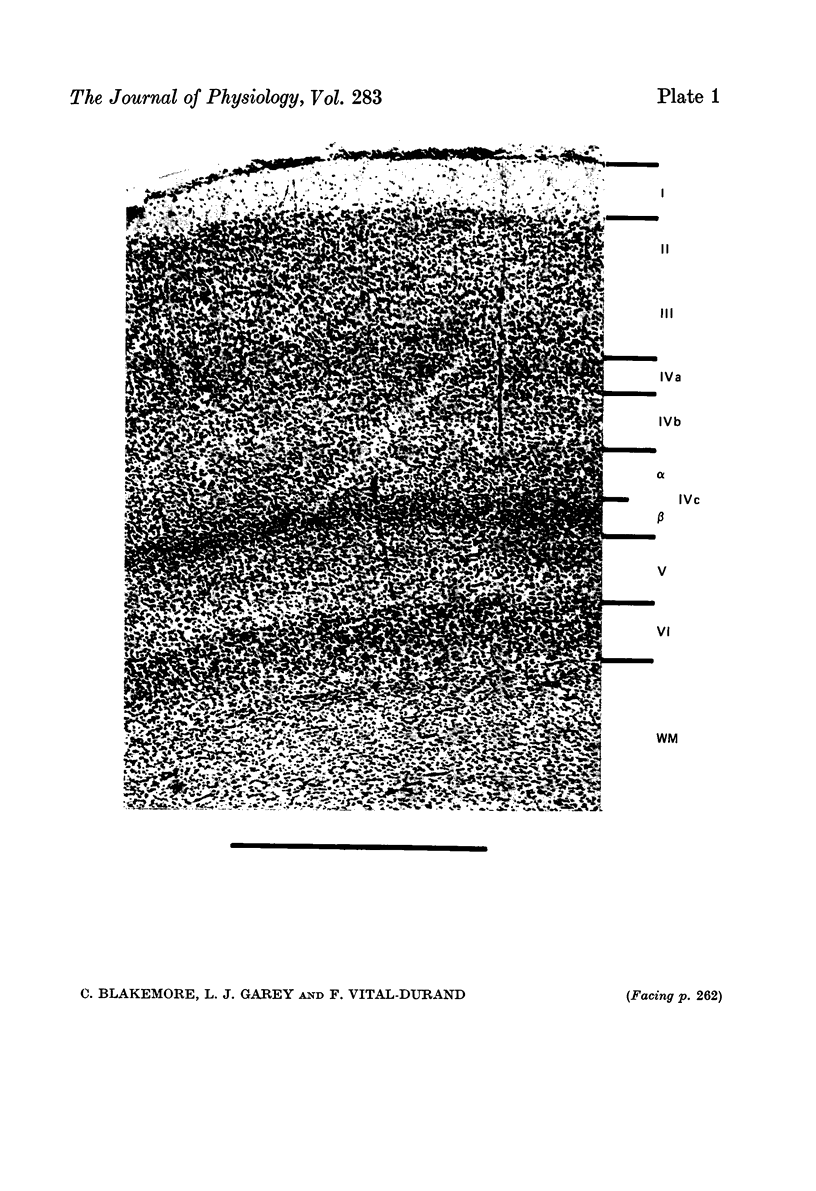
1. 1127 single units were recorded during oblique penetrations in area 17 of one normal, three monocularly deprived and four reverse sutured monkeys. 2. In all animals most cells outside layer IV c were orientation-selective, and preferred orientation usually shifted from cell to cell in a regular progressive sequence. 3. The presence in layer IV c of non-oriented, monocularly driven units, organized in alternating right-eye and left-eye 'stripes' (LeVay, Hubel & Wiesel, 1975) was confirmed. 4. Early monocular deprivation (2--5 1/2 weeks) caused a strong shift of ocular dominance towards the non-deprived eye. However, even outside layer IV c, neural background and some isolated cells could still be driven from the deprived eye in regularly spaced, narrow columnar regions. In layer IV c the non-deprived eye's stripes were almost three times wider, on average, than the deprived. 5. Later monocular deprivation (11--16 months) had no detectable influence on layer IV c but seemed to cause a small shift in ocular dominance outside IV c. Deprivation for 6 1/4 months in an adult had no such effect. 6. After early reverse suturing (at 5 1/2 weeks) the originally deprived eye gained dominance over cells outside layer IV c just as complete as that originally exercised by the eye that was first non-deprived. 7. The later reverse suturing was delayed, the less effective was recapture by the originally deprived eye. Reversal at 8 weeks led to roughly equal numbers of cells being dominated by each eye; fewer cells became dominated by the newly open eye after reverse suturing at 9 weeks and most of them were non-oriented; reversal at 38 1/2 weeks had no effect. 8. Binocular cells, though rare in reverse sutured animals, always had very similar preferred orientations in the two eyes. The columnar sequences of preferred orientation were not interrupted at the borders of ocular dominance columns. 9. Even within layer IV c there was evidence for re-expansion of physiologically determined ocular dominance stripes. After early reverse suture, stripes for the two eyes became roughly equal in width. Possible mechanisms for these changes are discussed.
Full text
PDF







































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertulis A., Guld C., Lennox-Buchthal M. A. Spectral and orientation specificity of single cells in foveal striate cortex of the vervet monkey, Cercopithecus aethiops. J Physiol. 1977 Jun;268(1):1–20. doi: 10.1113/jphysiol.1977.sp011843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Fiorentini A., Maffei L. A second neural mechanism of binocular depth discrimination. J Physiol. 1972 Nov;226(3):725–749. doi: 10.1113/jphysiol.1972.sp010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Garey L. J., Vital-Durand F. Reversal of physiological effects of monocular deprivation in monkeys [proceedings]. J Physiol. 1978 Mar;276:47P–49P. [PubMed] [Google Scholar]
- Blakemore C., Pettigrew J. D. Eye dominance in the visual cortex. Nature. 1970 Jan 31;225(5231):426–429. doi: 10.1038/225426a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Reversal of the physiological effects of monocular deprivation in kittens: further evidence for a sensitive period. J Physiol. 1974 Feb;237(1):195–216. doi: 10.1113/jphysiol.1974.sp010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisseret P., Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol. 1976 Feb;255(2):511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford M. L., Blake R., Cool S. J., von Noorden G. K. Physiological consequences of unilateral and bilateral eye closure in macaque monkeys: some further observations. Brain Res. 1975 Jan 24;84(1):150–154. doi: 10.1016/0006-8993(75)90809-4. [DOI] [PubMed] [Google Scholar]
- Dow B. M. Functional classes of cells and their laminar distribution in monkey visual cortex. J Neurophysiol. 1974 Sep;37(5):927–946. doi: 10.1152/jn.1974.37.5.927. [DOI] [PubMed] [Google Scholar]
- Dow B. M., Gouras P. Color and spatial specificity of single units in Rhesus monkey foveal striate cortex. J Neurophysiol. 1973 Jan;36(1):79–100. doi: 10.1152/jn.1973.36.1.79. [DOI] [PubMed] [Google Scholar]
- Dürsteler M. R., Garey L. J., Movshon J. A. Reversal of the morphological effects of monocular deprivation in the kittens's lateral geniculate nucleus. J Physiol. 1976 Sep;261(1):189–210. doi: 10.1113/jphysiol.1976.sp011553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. L., Schiller P. H., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. IV. Corticotectal cells. J Neurophysiol. 1976 Nov;39(6):1352–1361. doi: 10.1152/jn.1976.39.6.1352. [DOI] [PubMed] [Google Scholar]
- Gouras P. Opponent-colour cells in different layers of foveal striate cortex. J Physiol. 1974 May;238(3):583–602. doi: 10.1113/jphysiol.1974.sp010545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery R. W. Binocular competition in the control of geniculate cell growth. J Comp Neurol. 1972 Jan;144(1):117–129. doi: 10.1002/cne.901440106. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS OF CELLS IN STRIATE CORTEX OF VERY YOUNG, VISUALLY INEXPERIENCED KITTENS. J Neurophysiol. 1963 Nov;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol. 1972 Dec;146(4):421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J Comp Neurol. 1974 Dec 1;158(3):267–293. doi: 10.1002/cne.901580304. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., Stryker M. P. Orientation columns in macaque monkey visual cortex demonstrated by the 2-deoxyglucose autoradiographic technique. Nature. 1977 Sep 22;269(5626):328–330. doi: 10.1038/269328a0. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. T., Tucek P., McGowan M. A. Ocular fundus of the normal rhesus monkey. (Typical appearance from birth through 15 years). Vet Med Small Anim Clin. 1977 Apr;72(4):645–648. [PubMed] [Google Scholar]
- LeVay S., Hubel D. H., Wiesel T. N. The pattern of ocular dominance columns in macaque visual cortex revealed by a reduced silver stain. J Comp Neurol. 1975 Feb 15;159(4):559–576. doi: 10.1002/cne.901590408. [DOI] [PubMed] [Google Scholar]
- Lund J. S. Organization of neurons in the visual cortex, area 17, of the monkey (Macaca mulatta). J Comp Neurol. 1973 Feb 15;147(4):455–496. doi: 10.1002/cne.901470404. [DOI] [PubMed] [Google Scholar]
- Mansfield R. J. Neural basis of orientation perception in primate vision. Science. 1974 Dec 20;186(4169):1133–1135. doi: 10.1126/science.186.4169.1133. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Cynader M., Movshon J. A. Recovery from the effects of monocular deprivation in kittens. J Comp Neurol. 1977 Nov 1;176(1):53–63. doi: 10.1002/cne.901760104. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Blakemore C. Functional reinnervation in kitten visual cortex. Nature. 1974 Oct 11;251(5475):504–505. doi: 10.1038/251504a0. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Dürsteler M. R. Effects of brief periods of unilateral eye closure on the kitten's visual system. J Neurophysiol. 1977 Nov;40(6):1255–1265. doi: 10.1152/jn.1977.40.6.1255. [DOI] [PubMed] [Google Scholar]
- Movshon J. A. Reversal of the physiological effects of monocular deprivation in the kitten's visual cortex. J Physiol. 1976 Sep;261(1):125–174. doi: 10.1113/jphysiol.1976.sp011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A. The velocity tuning of single units in cat striate cortex. J Physiol. 1975 Aug;249(3):445–468. doi: 10.1113/jphysiol.1975.sp011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. I., Kato H., Bishop P. O. Discrimination of orientation and position disparities by binocularly activated neurons in cat straite cortex. J Neurophysiol. 1977 Mar;40(2):260–283. doi: 10.1152/jn.1977.40.2.260. [DOI] [PubMed] [Google Scholar]
- Olson C. R., Freeman R. D. Progressive changes in kitten striate cortex during monocular vision. J Neurophysiol. 1975 Jan;38(1):26–32. doi: 10.1152/jn.1975.38.1.26. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J Physiol. 1974 Feb;237(1):49–74. doi: 10.1113/jphysiol.1974.sp010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggio G. F., Baker F. H., Mansfield R. J., Sillito A., Grigg P. Spatial and chromatic properties of neurons subserving foveal and parafoveal vision in rhesus monkey. Brain Res. 1975 Dec 12;100(1):25–59. doi: 10.1016/0006-8993(75)90240-1. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Finlay B. L., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. I. Spatiotemporal organization of receptive fields. J Neurophysiol. 1976 Nov;39(6):1288–1319. doi: 10.1152/jn.1976.39.6.1288. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Finlay B. L., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. II. Orientation specificity and ocular dominance. J Neurophysiol. 1976 Nov;39(6):1320–1333. doi: 10.1152/jn.1976.39.6.1320. [DOI] [PubMed] [Google Scholar]
- Sherk H., Stryker M. P. Quantitative study of cortical orientation selectivity in visually inexperienced kitten. J Neurophysiol. 1976 Jan;39(1):63–70. doi: 10.1152/jn.1976.39.1.63. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Guillery R. W., Kaas J. H., Sanderson K. J. Behavioral, electrophysiological and morphological studies of binocular competition in the development of the geniculo-cortical pathways of cats. J Comp Neurol. 1974 Nov 1;158(1):1–18. doi: 10.1002/cne.901580102. [DOI] [PubMed] [Google Scholar]
- Spinelli D. N., Pribram K. H., Bridgeman B. Visual receptive field organization of single units in the visual cortex of monkey. Int J Neurosci. 1970 Oct;1(1):67–74. doi: 10.3109/00207457009147618. [DOI] [PubMed] [Google Scholar]
- Stryker M. P., Sherk H. Modification of cortical orientation selectivity in the cat by restricted visual experience: a reexamination. Science. 1975 Nov 28;190(4217):904–906. doi: 10.1126/science.1188372. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965 Nov;28(6):1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H., Lam D. M. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974 Oct 18;79(2):273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neurol. 1974 Dec 1;158(3):307–318. doi: 10.1002/cne.901580306. [DOI] [PubMed] [Google Scholar]
- Yates J. T. Chromatic information processing in the foveal projection (area striata) of unanesthetized primate. Vision Res. 1974 Feb;14(2):163–173. doi: 10.1016/0042-6989(74)90097-2. [DOI] [PubMed] [Google Scholar]
- von Noorden G. K. Experimental amblyopia in monkeys. Further behavioral observations and clinical correlations. Invest Ophthalmol. 1973 Oct;12(10):721–726. [PubMed] [Google Scholar]



