Abstract
A number of studies have indicated that Borrelia burgdorferi erp genes need not vary during vertebrate infection. However, it was recently reported that a B. burgdorferi bacterium reisolated from an infected mouse evidenced mutation and recombination events in several erp genes. Reexamination of that reisolate indicates that the previously reported changes were no doubt artifacts of the PCR processes originally used to clone those DNAs. Thus, no evidence has been found of erp gene variation during mammalian infection.
All examined isolates of the Lyme disease spirochete, Borrelia burgdorferi, contain numerous different circular plasmids of approximately 32 kb in size, the cp32 family (30). As many as 10 different cp32 plasmid family members have been identified in clonal populations of B. burgdorferi (30). These plasmids are largely homologous, varying significantly at only three loci: two separate lipoprotein-encoding loci (the erp and 2.9 loci) and the plasmid segregation protein-encoding genes (30). Apparently, it is differences among the segregation proteins that account for the ability of so many different cp32 plasmids to reside within a single bacterial cell, since plasmids with similar segregation genes are incompatible (6, 20).
Erp proteins are surface-exposed outer membrane proteins that are produced during the initial stages of mammalian infection, and a single bacterium can express its entire Erp repertoire simultaneously (7, 9, 11, 16, 30). The cp32 erp loci have been given various other names by different researchers, including ospE, ospF, elp, p21, bbk2.10, bbk2.11, pG, and “upstream homology box genes” (30). The many erp genes found within an individual bacterium often exhibit a considerable range of sequence variation. For example, the B. burgdorferi type strain B31 is known to contain 17 erp genes, arranged in 10 separate loci, that encode proteins having primary sequences that range between 16 and 100% identity (3, 4, 26). This sequence variability has arisen, at least in part, from recombination among erp genes, although it is not yet known at which point(s) in the infection cycle such recombination occurs (27).
Lyme disease spirochetes can persistently infect immunocompetent warm-blooded animals for extensive periods of time. The mechanism(s) by which B. burgdorferi avoids clearance by the host immune system is yet to be fully elucidated. A number of pathogenic microorganisms, such as the relapsing fever borreliae and the malaria plasmodia, utilize genetic variation mechanisms that constantly produce novel surface proteins, permitting the pathogen to remain a step ahead of host antibody production (15, 23, 31). Lyme disease borreliae possess at least one such genetic variation mechanism, the vlsE system (35).
When the erp genes were first discovered in B. burgdorferi, the multiplicity of these genes, coupled with the variations observed between different strains, suggested that they may also constitute an antigenic variation mechanism that facilitates chronic vertebrate infection. However, three separate studies have demonstrated that erp variation is not required for persistent infection of immunocompetent animals. In each of those studies, laboratory animals were infected with a clonal B. burgdorferi culture and then bacteria were reisolated from infected tissues after up to 1 year of infection. erp gene sequences of these reisolates were identical to those of each clonal inoculant strain (8, 10, 18, 32). Other experimental evidence adds to the results of those studies. erp genes have been sequenced from at least five different cultures of strain B31, all of which had been passaged through different immunocompetent mice at least once prior to analysis, and in all but one case, erp gene sequences have been absolutely identical. The only known difference is a single point mutation in the erpB gene of a high-passage-number, noninfectious culture that has lost many plasmids and acquired several other mutations (3, 4, 26, 29). Together, these data indicate that variation among erp genes serves a function other than evasion of host immune responses during persistent vertebrate infection.
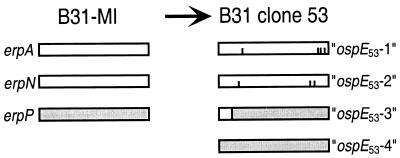
It was of great interest when a recent report announced evidence of recombination and other mutational events in erp genes during mammalian infection (32). In that study, mice were infected with a clonal culture of B. burgdorferi strain B31-MI (referred to as B31G in that report), and 3 months later, bacteria were reisolated, cultured, and cloned by plating. Oligonucleotides designed to amplify three of the erp genes were then used in PCR of each clonal reisolate, following which the amplicons were cloned into plasmids in Escherichia coli and sequenced. The PCR products of one such reisolate, clone 53, contained sequences different from those of the original B31-MI inoculant (32). The erp genes examined in that study all share greater than 80% sequence identity to the ospE gene of strain N40 and were therefore referred to as ospE genes (32). Strain B31-MI contains three such genes, with the formal designations erpA (BBP38, on cp32-1), erpN (BBL39, on cp32-8), and erpP (BBN38, on cp32-9) (3, 4, 26, 29). The erpA and erpN genes are identical, while erpP shares approximately 87% identical nucleotides with the other two genes (3). Reisolate clone 53 appeared to be especially remarkable, in that this bacterium was reported to contain four ospE-like genes (Fig. 1) (32). One gene was identical to erpP, and two differed from erpA and erpN by either 3- or 4-bp changes, while the fourth was a chimera of erpA or -N and erpP. As noted above, plasmids with identical segregation genes are incompatible. Thus, it appeared that clone 53 contains not only mutant erp genes but that one of the original genes must have been duplicated, mutated, and moved to a novel location. Richard Marconi (Virginia Commonwealth University, Richmond) kindly provided a low-passage-number culture of B31 clone 53 to permit further characterization of this bacterium.
FIG. 1.
Schematic of the ospE-like erp genes of B. burgdorferi strain B31-MI (3) and those reported for reisolate clone 53 (32). The B31-MI erpA and erpN genes are identical, while the sequence of erpP is somewhat different. The reported clone 53 genes ospE53-1 and ospE53-2 are identical to erpA and erpN with the exception of four and three single-nucleotide substitutions, respectively (indicated by vertical lines). The reported clone 53 gene ospE53-3 is a chimera of either erpA or erpN with erpP. The clone 53 gene ospE53-4 is identical to the B31-MI erpP gene.
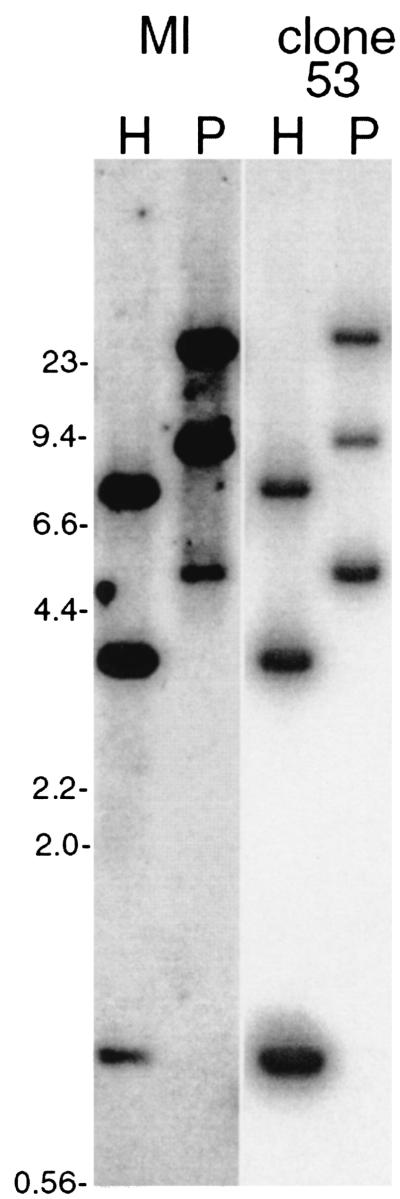
Confirmation of this unique genetic phenomenon was first addressed by Southern blot analyses of digested DNAs from both B31-MI and clone 53. Bacteria were grown to densities of approximately 108 bacteria per ml in modified Barbour-Stoenner-Kelly medium (BSK-H; Sigma, St. Louis, Mo.). Cultures were harvested by centrifugation, and plasmids were purified using Qiagen mini kits (Qiagen, Valencia, Calif.). Due to the extensive homologies among cp32 plasmids, very few restriction endonucleases generate unique digestion patterns from multiple plasmids (4). Analysis of the sequences of B31-MI cp32 plasmid family members (GenBank accession numbers AE001575 through AE001581 and AE001584) revealed that digestion with either HpaII or PstI will yield uniquely sized DNA fragments that each contain one of that strain's erp loci. None of the erp genes examined in this study contains HpaII or PstI sites. Total plasmid DNAs from B31-MI and B31 clone 53 were digested with either of those two enzymes, separated by pulsed-field agarose gel electrophoresis, and blotted to a nylon membrane (8). A DNA probe was produced by PCR from an E. coli recombinant plasmid clone of erpP (pBLS538), using oligonucleotides AINP5′ and ACINP3′ (Table 1), with reaction conditions consisting of 20 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min. The amplicon was diluted 100-fold in water and amplified a second time, and an aliquot was analyzed by agarose gel electrophoresis for purity. The resulting DNA was purified through a Centricon-100 microconcentrator (Amicon, Beverly, Mass.) and was labeled with [α-32P]dATP by random priming (Life Technologies, Gaithersburg, Md.). This probe was hybridized with the membrane overnight at 55°C in 6× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate-5 g of nonfat dried milk per liter. The following day the blot was washed extensively with 0.2× SSC-0.1% sodium dodecyl sulfate at 55°C (high-stringency wash conditions). Hybridized bands were detected by autoradiography. Both B31-MI and clone 53 exhibited identical HpaII and PstI restriction patterns, with three autoradiography bands of the predicted sizes detected from each (Fig. 2). These data indicate that either the conclusion of four ospE-like genes in clone 53 was incorrect or that the postulated duplication-mutation-translocation event occurred in a DNA segment having final HpaII and PstI restriction patterns identical to those of a parental erp locus.
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′ to 3′) |
|---|---|
| AINP5′ | ATGCTCGAGAAGATTCATACTTCATATGATGAG |
| ACINP3′ | TATGGATCCTCTCTCCTATATTTCTAACTTC-3′ |
| CL53-1 | AGCAAAGCAATGGAGAGGTAAAGG |
| CL53-2 | CTTTGTTTTGGATACCATTTGCAC |
| E-142 | CTAGTGATATTGCATATTCAG |
| D-1 | ACGATAGGGTAATATCAAAAAAGG |
| CP8-1 | GAAGATTTAAACAAAAAAATTGCG |
FIG. 2.
Southern blot analysis of B31-MI and clone 53 plasmid DNAs digested with either HpaII or PstI (lanes H or P, respectively) and hybridized with a radiolabeled probe derived from the B31-MI erpP gene. DNA fragment sizes were as predicted from analysis of the B31-MI genome (see text). In order of descending size, HpaII fragments correspond with erpA (cp32-1, 7,540 bp), erpN (cp32-8, 3,631 bp), and erpP (cp32-9, 1,014 bp), while PstI fragments correspond with erpN (24,725 bp), erpA (9,452 bp), and erpP (5,073 bp). DNA size standards are indicated to the left (in kilobases).
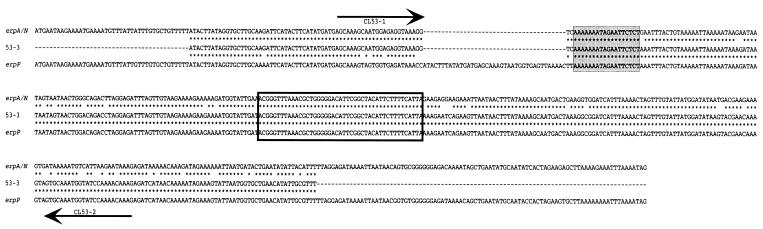
The erp genes of B31 clone 53 were further analyzed by PCR using gene-specific primers. The sequence reported as gene ospE53-3 is a chimera of either erpA or erpN with erpP (Fig. 1 and 3). Unique sequences found in this chimera enabled production of ospE53-3-specific PCR oligonucleotide primers CL53-1, complementary to the erpA/erpN 5′ end, and CL53-2, complementary to the erpP 3′ end (Table 1; Fig. 3). Both B31-MI and B31 clone 53 plasmid DNAs were subjected to PCR, with conditions consisting of 20 cycles of 94°C for 1 min, 50°C for 30 s, and 72°C for 1 min. Repeated attempts all failed to produce an amplicon from clone 53, although these reaction conditions are used routinely in our laboratory to specifically amplify other B. burgdorferi DNA fragments (data not shown). Less stringent PCR conditions were also utilized, consisting of 25 cycles of 94°C for 1 min, 50°C for 1 min, and 65°C for 1 min. Five separate reactions yielded products from clone 53 DNA only three times. Additionally, PCR of B31-MI, which lacks an erpA/N::erpP chimera (3), yielded amplicons from two of four separate reactions.
FIG. 3.
Alignment of the B31-MI erpA/N and erpP genes with the reported sequence of the clone 53 ospE53-3 gene (labeled 53-3). Identical nucleotides are indicated by asterisks. The site of the DNA strand exchange event that would have given rise to ospE53-3 is indicated by a shaded box. ospE53-3 is identical to erpA/N 5′ of this location and identical to erpP 3′ of this site. The location of the DNA strand exchange event that gave rise to the novel PCR artifactual chimera generated during the present studies is indicated by an unshaded box. The DNA sequences complementary to PCR oligonucleotides are indicated by arrows above or below the sequence, with arrowheads indicating their 5′-to-3′ direction.
The nature of the above-described amplification products was addressed by DNA sequencing of both uncloned and cloned amplicons. Most products were determined to have resulted from misprimed PCR, with one oligonucleotide or the other having primed from an incompletely matched DNA site. Six of 9 analyzed B31-MI amplicons contained erpA/N genes, one contained an erpP gene, and two were chromosomal fragments. Sequencing of uncloned amplicons from clone 53 identified only nonmutated erpA/N or erpP genes, with no evidence of a chimera. Examination of five cloned amplicons from this bacterium revealed that three consisted of nonmutated erpA/N genes and that one consisted of a nonmutated erpP gene, while the fifth clone contained a chimeric gene. However, this chimera was unlike that reported as ospE53-3, with the apparent strand-crossing event having occurred at a different location (Fig. 3).
The above data indicate that the earlier conclusions regarding ospE53-3 were most likely incorrect. Southern blot analysis indicated identical restriction endonuclease digestion patterns of both the parent and reisolated clone, and PCR failed to amplify an ospE53-3 sequence from clone 53 DNA despite repeated attempts. The only other possible explanation for these results is that every bacterium in the studied clone 53 culture has lost the ospE53-3 locus. While B. burgdorferi may lose plasmids during in vitro cultivation, the analyzed clone 53 was grown in culture for a very limited number of generations since reisolation. Unless another culture of clone 53 can be found and conclusively demonstrated to contain an ospE53-3 sequence, it is most reasonable to conclude that the previously reported sequence was a PCR artifact.
PCR is notorious for producing such artifacts, which arise from strand exchange during amplification of a mixture of similar DNA templates (1, 19, 21, 22, 24, 25, 33). As one example, rRNA gene PCR analysis of a mixture of defined bacterial species yielded at least 32% aberrant amplicons (34). Strand exchange during PCR also best explains the novel erp chimera obtained in the present study: as with ospE53-3, it is extremely unlikely that this previously undetected chimera arose from a recombination event accompanied by duplication and translocation into a DNA segment that has a restriction pattern identical to that of a parental erp locus and that can only rarely be amplified from clone 53 DNA.
Sequencing of the misprimed clone 53 amplicons indicated that, in addition to the nonmutated erpP gene previously reported as being present (32), these bacteria also contain at least one nonmutated erpA/erpN gene. Yet the earlier report stated that the two genes in clone 53 most similar to erpA and erpN, ospE53-1 and ospE53-2, both contain point mutations (32). To clarify this discrepancy, both the erpA and erpN loci of clone 53 were specifically analyzed. While the erpA and erpN genes of the starting strain, B31-MI, are identical, they are carried by different plasmids, each of which possesses a unique set of plasmid segregation genes (3, 27). Thus, each of these two erp loci can be specifically PCR amplified using an oligonucleotide complementary to the shared DNA sequences 3′ of the erp locus (E-142 [Table 1]), and a specific oligonucleotide complementary to the appropriate plasmid segregation locus (D-1 and CP8-1, for cp32-1 and cp32-8, respectively) (3, 4, 27). PCR conditions consisted of 10 cycles of 94°C for 10 s, 50°C for 30 s, and 68°C for 8 min, followed by 20 cycles starting with 94°C for 10 s, 50°C for 30 s, and 68°C for 8 min with successive extension steps increased by 20 s each, using an Expand PCR amplification system (Boehringer-Mannheim, Indianapolis, Ind.). Amplicons were purified using Centricon-100 microconcentrators and were partially sequenced. Both amplification reactions were separately performed two times. Both the erpA and erpN genes of clone 53 proved to be identical to those of the parent B31-MI. The PCR process is well known for introducing nucleotide substitutions, especially when using a nonproofreading DNA polymerase such as the Taq enzyme utilized in the earlier study (2, 5, 13, 17). It was for this reason that the present study examined the sequences of multiple, independently produced amplicons, rather than a single, cloned PCR product as with the previous report. These data indicate that the clone 53 DNA sequences reported as ospE53-1 and ospE53-2 were also likely to have been PCR artifacts.
It is therefore concluded that all of the “mutant” erp genes reported to be present in clone 53 can be discounted as PCR artifacts and that they are not legitimate genetic variants that arose during mammalian infection. The same report described four parallel reisolate clones containing point mutations in either the erpA, erpN, or erpP gene, each also identified through sequence analysis of single PCR amplicons (32). While those four reisolates were not studied here, the results of the present analysis of clone 53 strongly suggest that those other reported variants may also be artifactual.
In summary, these and other studies indicate that B. burgdorferi erp genes remain stable during vertebrate infection. Many, if not all, Erp proteins bind mammalian complement inhibitor factor H and thus likely protect the bacteria against complement-mediated killing in vertebrate hosts (12, 28). Erp protein amino acid sequence variations affect their relative affinities for the complement factor H of different potential vertebrate hosts, which is proposed to contribute to the broad host range of Lyme disease spirochetes (14, 28). This function of Erp proteins suggests a possible reason for erp gene stability during vertebrate infection: mutation of a gene encoding an essential factor H-binding Erp might be lethal to the bacterium.
Acknowledgments
This study was funded by Public Health Service grant RO1-AI44254.
I thank Richard Marconi for providing B. burgdorferi B31 clone 53 and for constructive discussions of these results; Kelly Babb, Nazira El-Hage, Melissa Hines, Natalie Mickelsen, and Jennifer Miller for technical assistance; and Sherwood Casjens, Patti Rosa, and Wolf Zückert for comments on this study and the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Bracho, M. A., A. Moya, and E. Barrio. 1998. Contribution of Taq polymerase-induced errors to the estimation of RNA virus diversity. J. Gen. Virol. 79:2921-2928. [DOI] [PubMed] [Google Scholar]
- 2.Cariello, N. F., J. A. Swenberg, and T. R. Skopek. 1991. Fidelity of Thermococcus litoralis DNA polymerase (Vent) in PCR determined by denaturing gradient gel electrophoresis. Nucleic Acids Res. 11:4193-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs of an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 4.Casjens, S., R. van Vugt, K. Tilly, P. A. Rosa, and B. Stevenson. 1997. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J. Bacteriol. 179:217-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cline, J., J. C. Braman, and H. H. Hogrefe. 1996. PCR fidelity of Pfu DNA polymerase and other thermostable DNA polymerases. Nucleic Acids Res. 24:3546-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggers, C. H., M. J. Caimano, M. L. Clawson, W. G. Miller, D. S. Samuels, and J. D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43:281-295. [DOI] [PubMed] [Google Scholar]
- 7.El-Hage, N., K. Babb, J. A. Carroll, N. Lindstrom, E. R. Fischer, J. C. Miller, R. D. Gilmore, Jr., M. L. Mbow, and B. Stevenson. 2001. Surface exposure and protease insensitivity of Borrelia burgdorferi Erp (OspEF-related) lipoproteins. Microbiology 147:821-830. [DOI] [PubMed] [Google Scholar]
- 8.El-Hage, N., L. D. Lieto, and B. Stevenson. 1999. Stability of erp loci during Borrelia burgdorferi infection: recombination is not required for chronic infection of immunocompetent mice. Infect. Immun. 67:3146-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Hage, N., and B. Stevenson. Simultaneous coexpression of Borrelia burgdorferi Erp proteins occurs through a specific erp locus-directed regulatory mechanism. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 10.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, and D. R. Akins. 2002. Changes in temporal and spatial patterns of outer surface lipoprotein expression generate population heterogeneity and antigenic diversity in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 70:3468-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, J. D. Radolf, and D. R. Akins. 2001. Regulation of OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect. Immun. 69:3618-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellwage, J., T. Meri, T. Heikkilä, A. Alitalo, J. Panelius, P. Lahdenne, I. J. T. Seppälä, and S. Meri. 2001. The complement regulatory factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 13.Keohavong, P., and W. G. Thilly. 1989. Fidelity of DNA polymerases in DNA amplification. Proc. Natl. Acad. Sci. USA 86:9253-9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtenbach, K., S. DeMichelis, S. Etti, S. M. Schäfer, H.-S. Sewell, V. Brade, and P. Kraiczy. 2002. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol. 10:74-79. [DOI] [PubMed] [Google Scholar]
- 15.Kyes, S., P. Horrocks, and C. Newbold. 2001. Antigenic variation at the infected red cell surface in malaria. Annu. Rev. Microbiol. 55:673-707. [DOI] [PubMed] [Google Scholar]
- 16.Lam, T. T., T.-P. K. Nguyen, R. R. Montgomery, F. S. Kantor, E. Fikrig, and R. A. Flavell. 1994. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect. Immun. 62:290-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loewen, P. C., and J. Switala. 1995. Template secondary structure can increase the error frequency of the DNA polymerase from Thermus aquaticus. Gene 164:59-63. [DOI] [PubMed] [Google Scholar]
- 18.McDowell, J. V., S. Y. Sung, G. Price, and R. T. Marconi. 2001. Demonstration of the genetic stability and temporal expression of select members of the Lyme disease spirochete OspF protein family during infection in mice. Infect. Immun. 69:4831-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyerhans, A., J. P. Vartanian, and S. Wain-Hobson. 1990. DNA recombination during PCR. Nucleic Acids Res. 11:1687-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novick, R. P. 1987. Plasmid incompatibility. Microbiol. Rev. 51:381-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pääbo, S., D. M. Irwin, and A. C. Wilson. 1990. DNA damage promotes jumping between templates during enzymatic amplification. J. Biol. Chem. 265:4718-4721. [PubMed] [Google Scholar]
- 22.Qiu, X., L. Wu, H. Huang, P. E. McDonel, A. V. Palumbo, J. M. Tiedje, and J. Zhou. 2001. Evaluation of PCR-generated chimeras, mutations, and heteroduplexes with 16S rRNA gene-based cloning. Appl. Environ. Microbiol. 67:880-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Restrepo, B. I., and A. G. Barbour. 1994. Antigen diversity in the bacterium B. hermsii through “somatic” mutations in rearranged vmp genes. Cell 78:867-876. [DOI] [PubMed] [Google Scholar]
- 24.Shuldiner, A. R., A. Nirula, and J. Roth. 1989. Hybrid DNA artifact from PCR of closely related target sequences. Nucleic Acids Res. 17:4409.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speksnijder, A. G. C. L., G. A. Kowalchuk, S. De Jong, E. Kline, J. R. Stephen, and H. J. Laanbroek. 2001. Microvariation artifacts introduced by PCR and cloning of closely related 16S rRNA gene sequences. Appl. Environ. Microbiol. 67:469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson, B., J. L. Bono, T. G. Schwan, and P. Rosa. 1998. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect. Immun. 66:2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevenson, B., S. Casjens, and P. Rosa. 1998. Evidence of past recombination events among the genes encoding the Erp antigens of Borrelia burgdorferi. Microbiology 144:1869-1879. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevenson, B., K. Tilly, and P. A. Rosa. 1996. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J. Bacteriol. 178:3508-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevenson, B., W. R. Zückert, and D. R. Akins. 2001. Repetition, conservation, and variation: the multiple cp32 plasmids of Borrelia species, p. 87-100. In M. H. Saier and J. García-Lara (ed.), The spirochetes: molecular and cellular biology. Horizon Press, Oxford, United Kingdom. [PubMed]
- 31.Stoenner, H. G., T. Dodd, and C. Larsen. 1982. Antigenic variation in B. hermsii. J. Exp. Med. 156:1297-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sung, S. Y., J. V. McDowell, J. A. Carlyon, and R. T. Marconi. 2000. Mutation and recombination in the upstream homology box-flanked ospE-related genes of the Lyme disease spirochetes result in the development of new antigenic variants during infection. Infect. Immun. 68:1319-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, G. C.-Y., and Y. Wang. 1996. The frequency of chimeric molecules as a consequence of PCR co-amplification of 16S rRNA genes from different bacterial species. Microbiology 142:1107-1114. [DOI] [PubMed] [Google Scholar]
- 34.Wang, G. C.-Y., and Y. Wang. 1997. Frequency of formation of chimeric molecules as a consequence of PCR coamplification of 16S rRNA genes from mixed bacterial genomes. Appl. Environ. Microbiol. 63:4645-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, J.-R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:1-20. [DOI] [PubMed] [Google Scholar]