Abstract
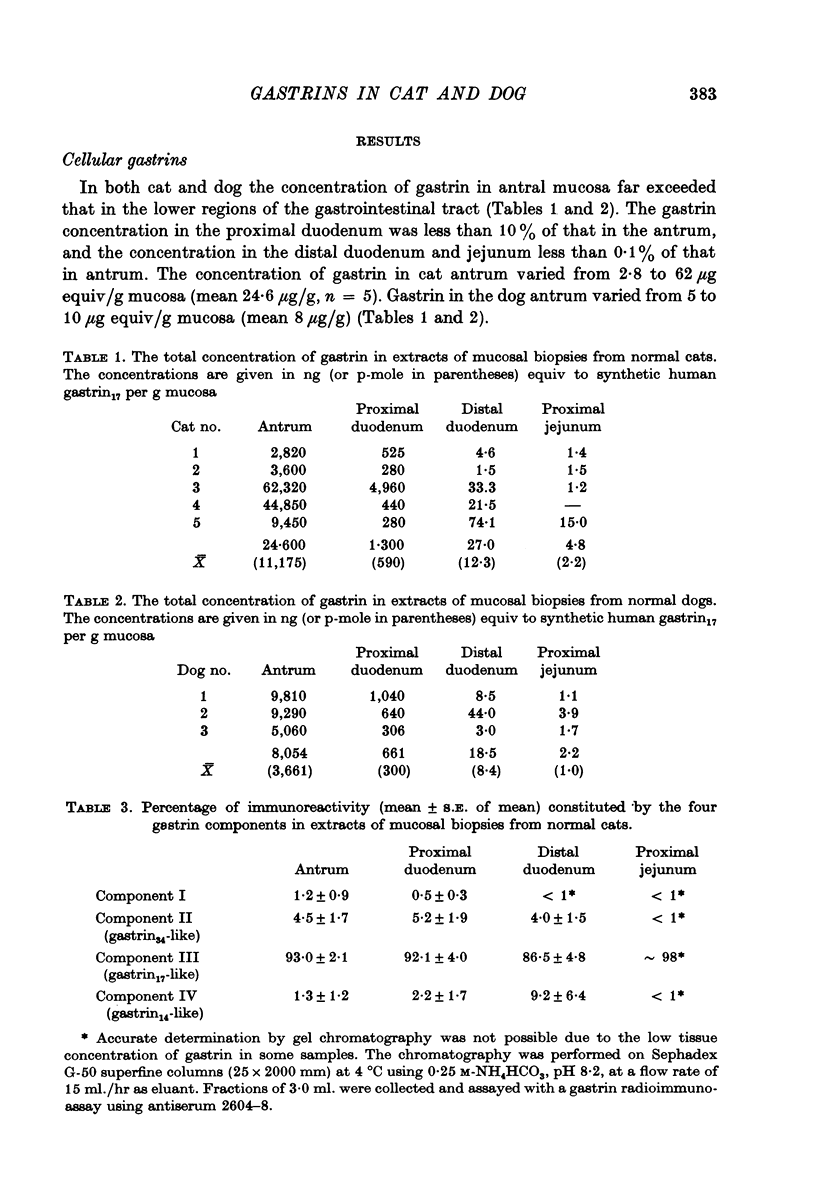
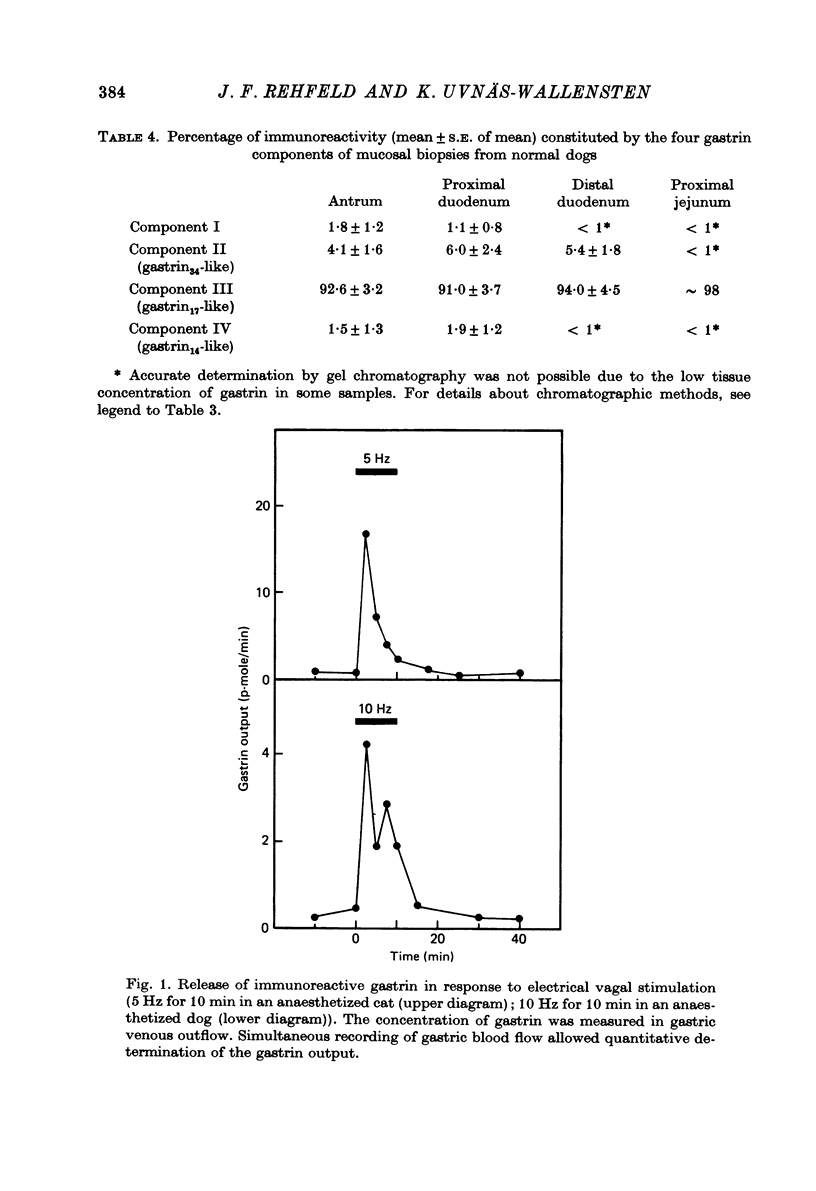
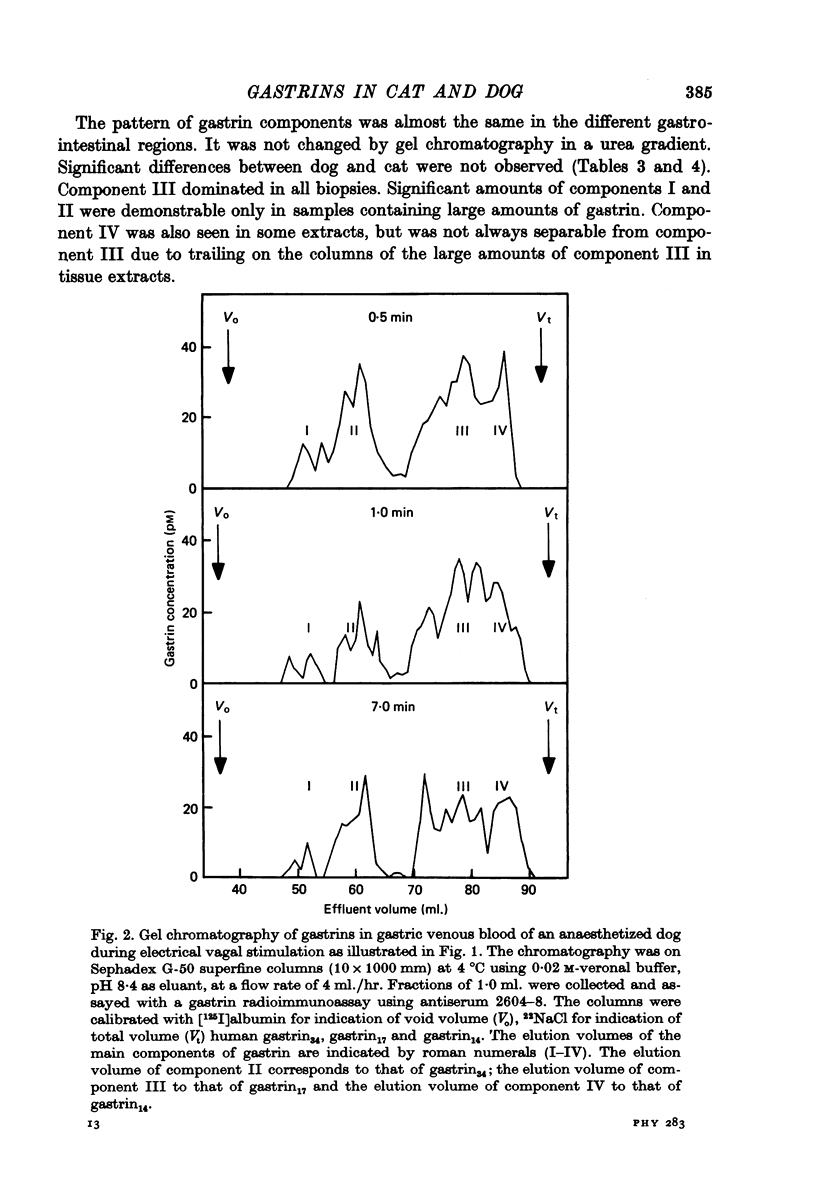
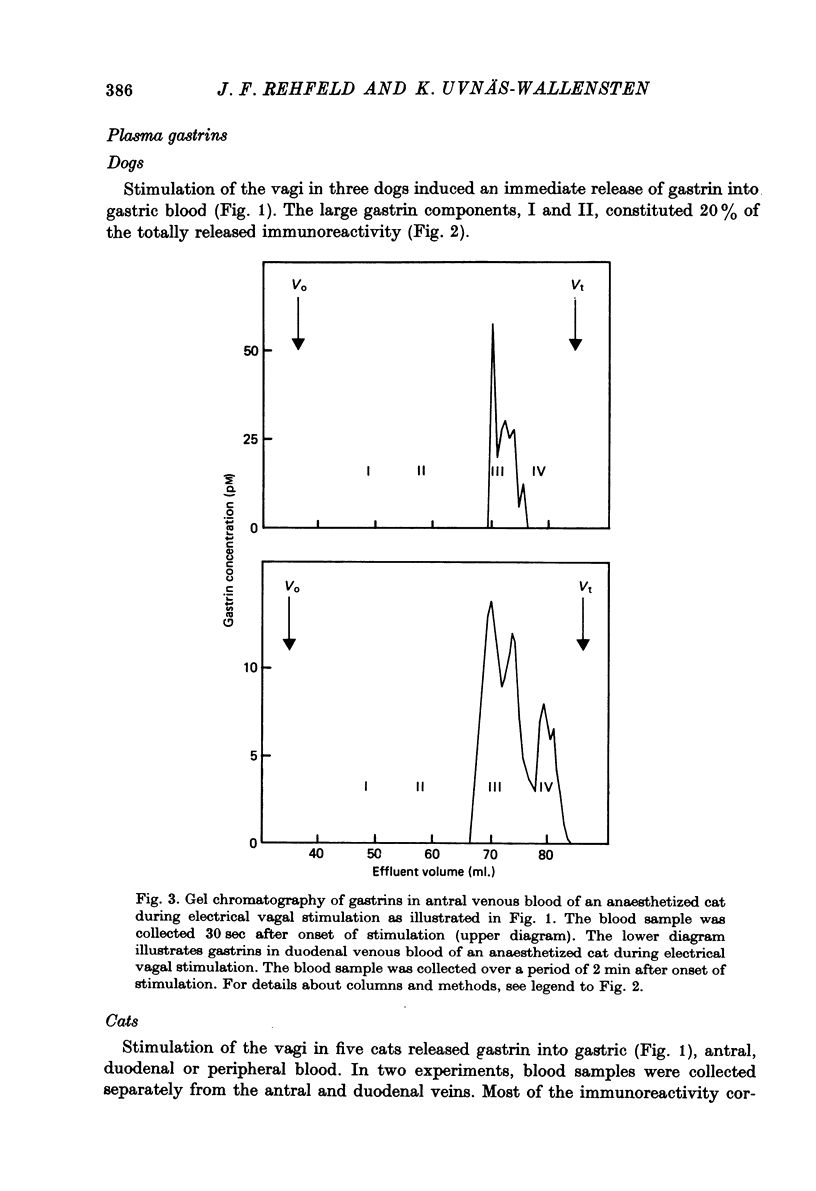
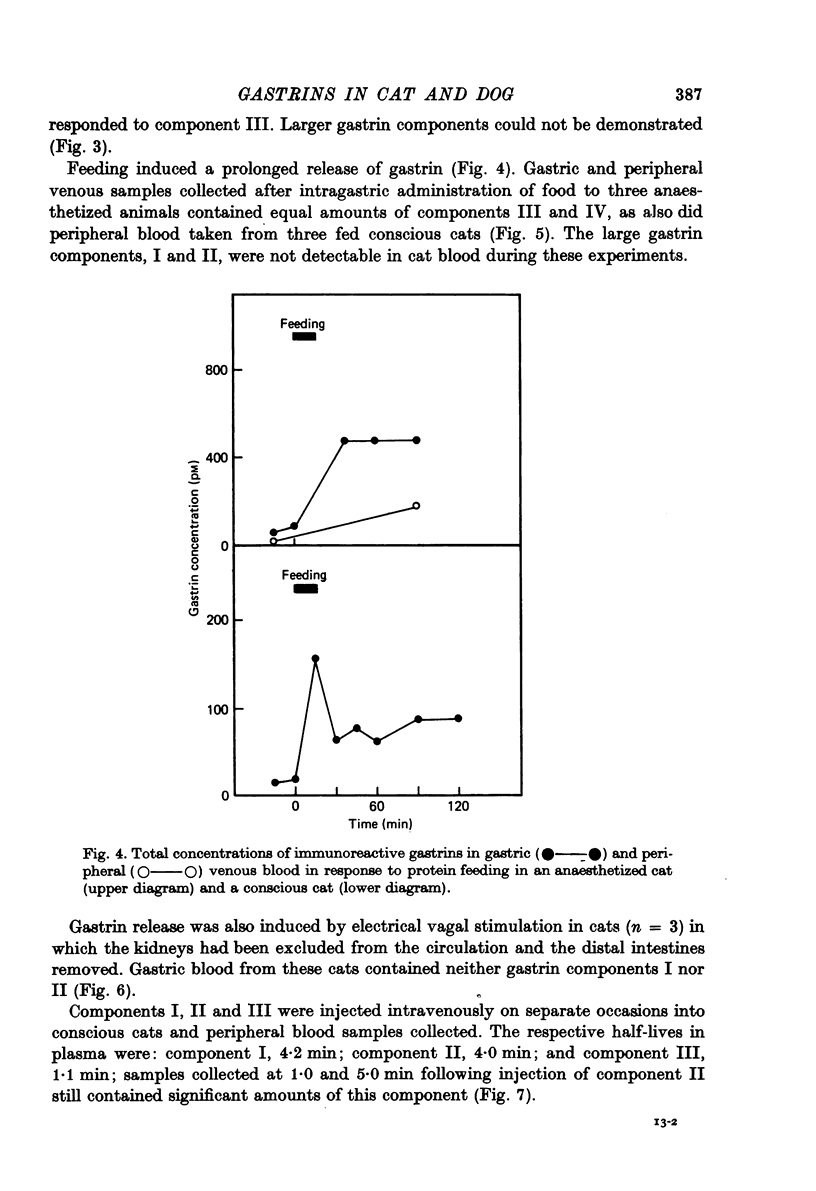
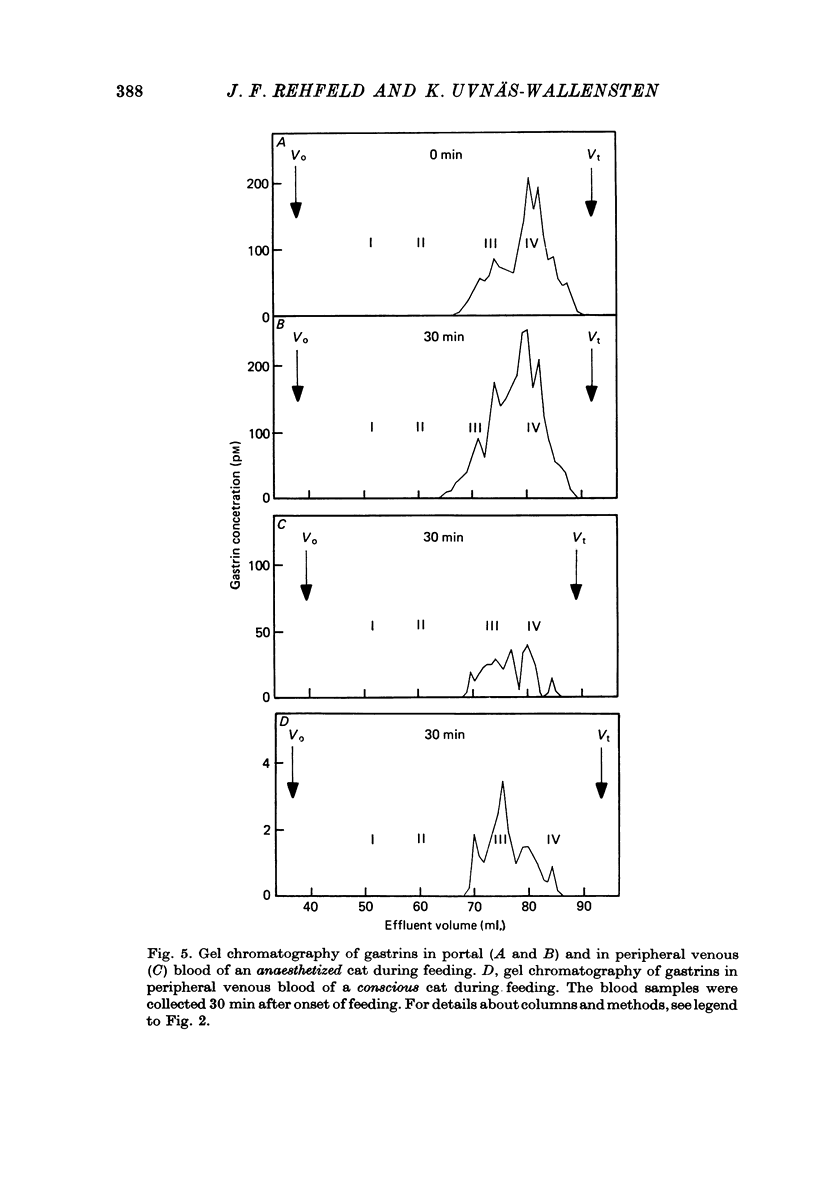
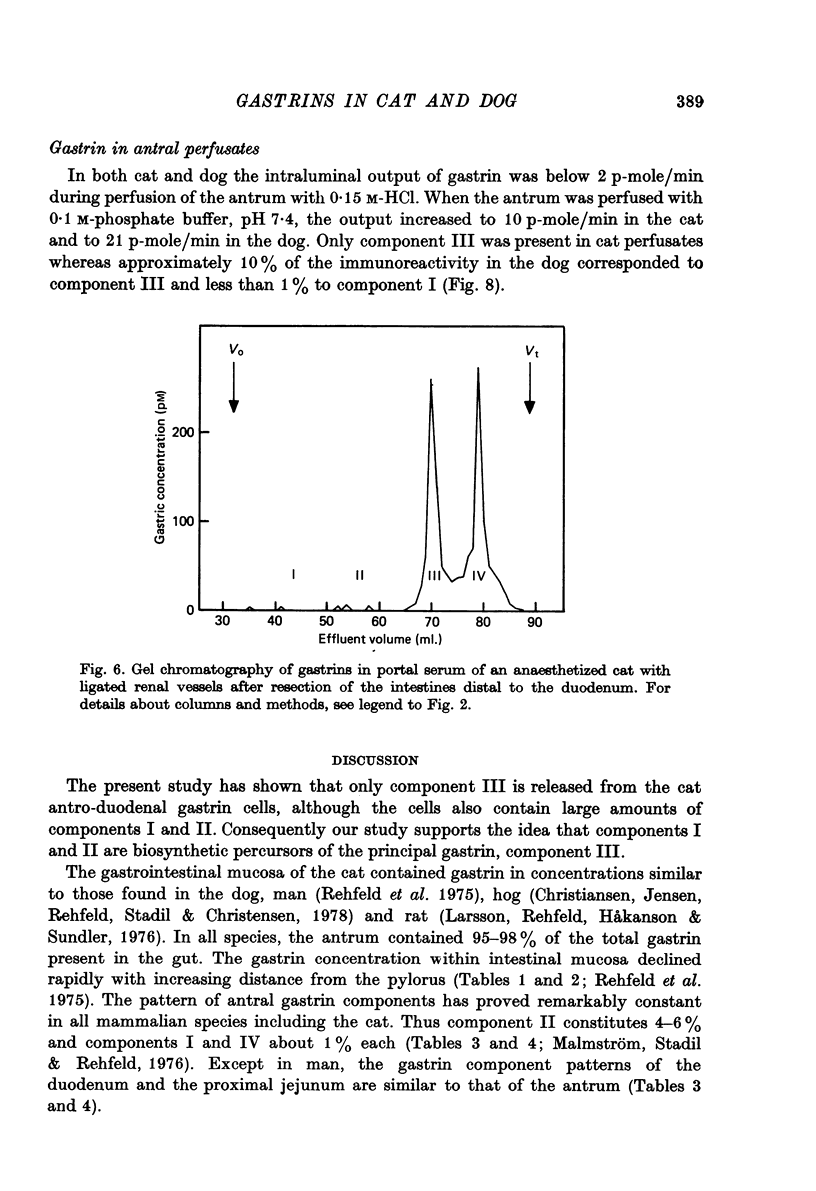
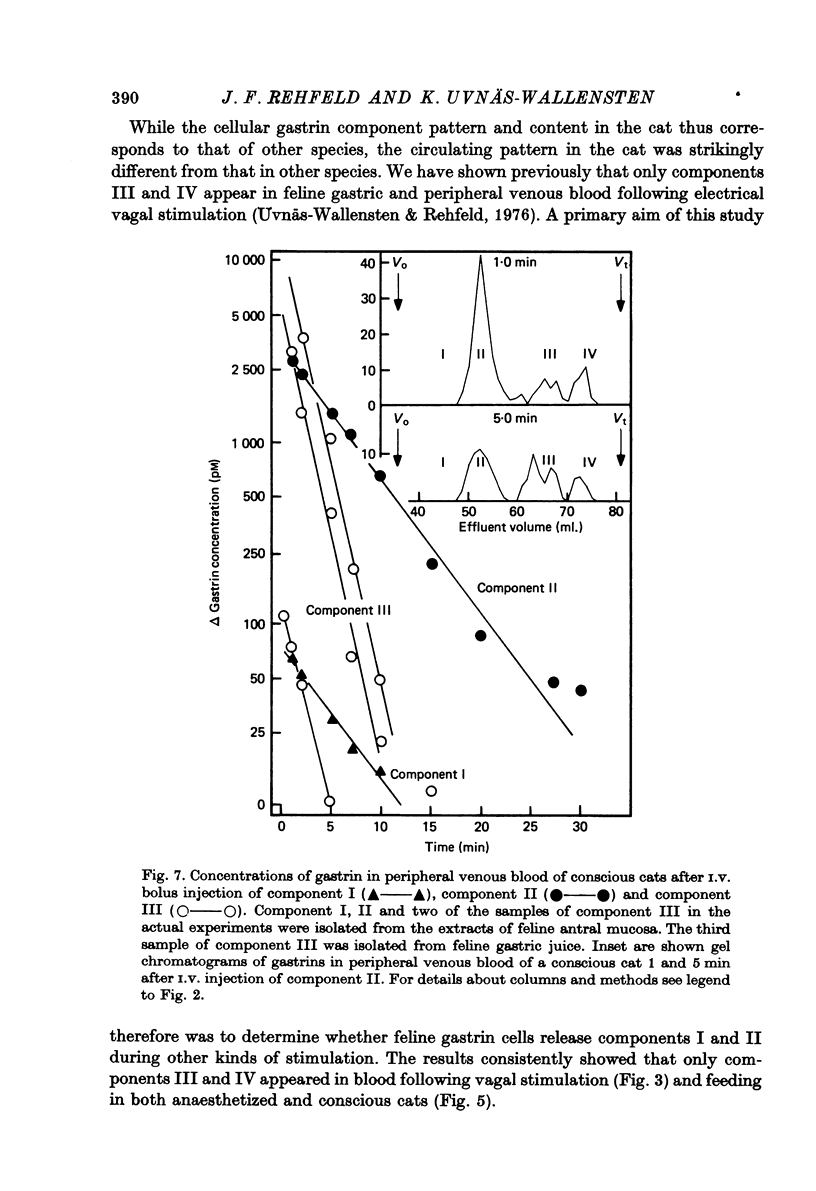
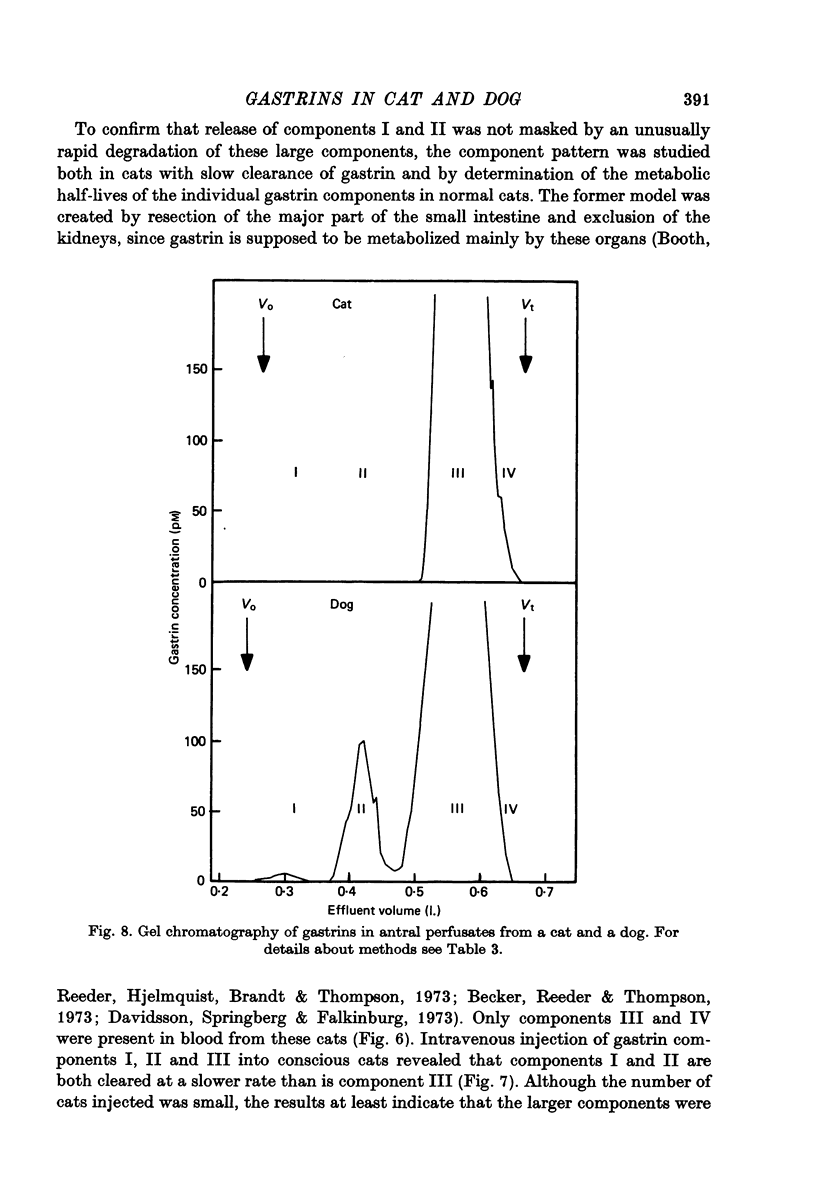
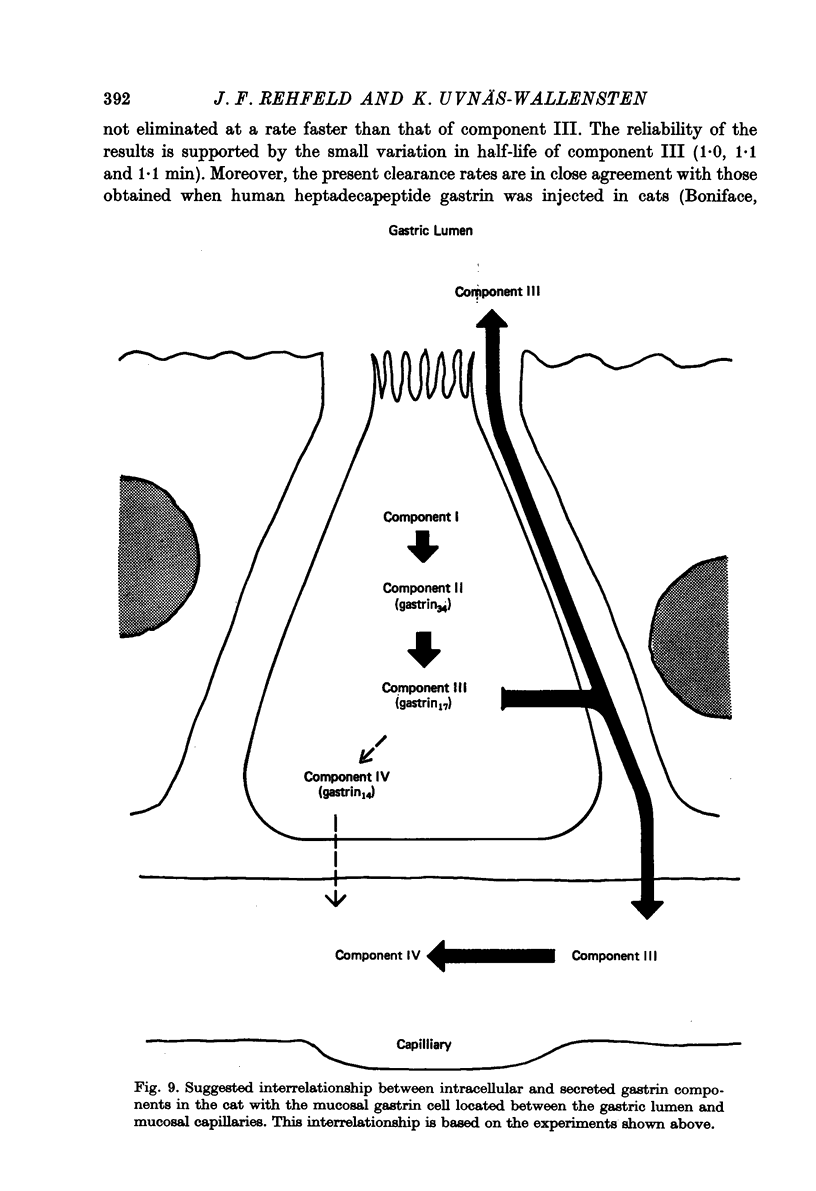
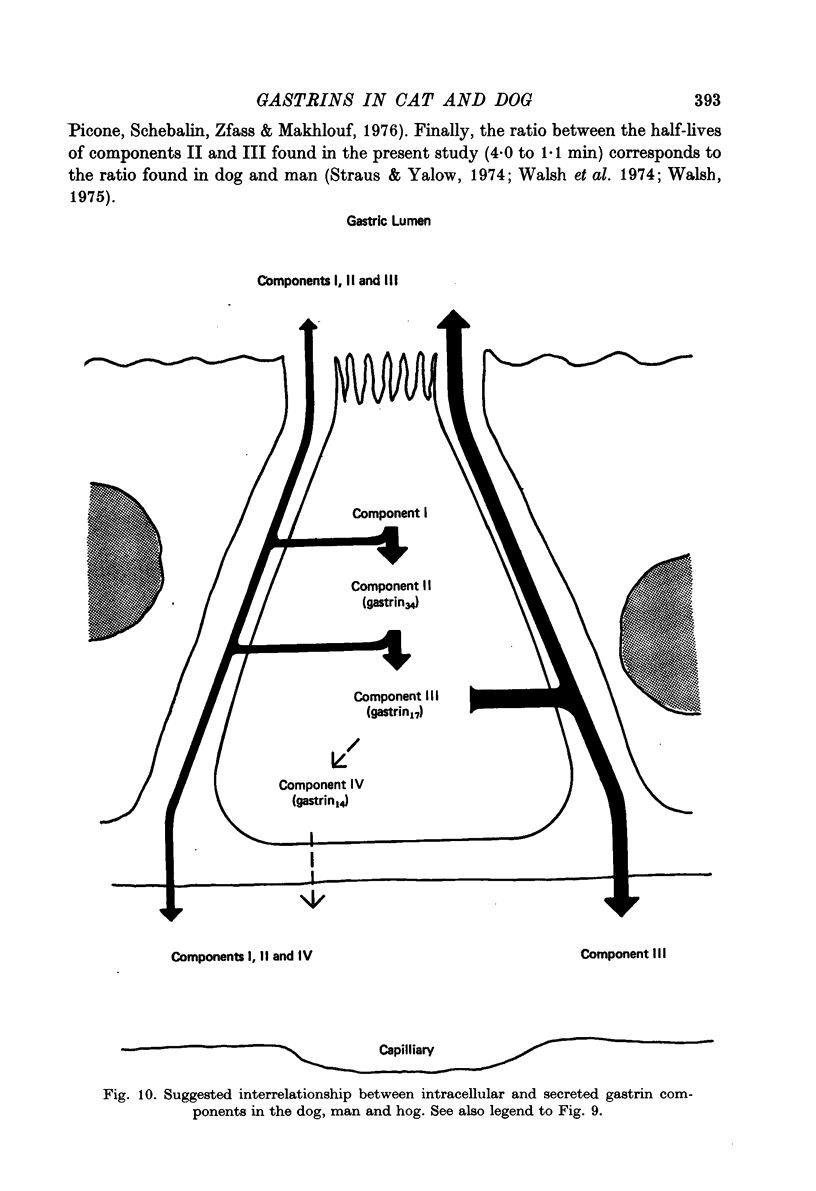
1. Extracts of antral, duodenal and jejunal mucosa contained the same concentrations and molecular forms of gastrin in cat and dog. In both species component III constituted 93%, component II 4% and components I and IV each 1% of the total immunoreactivity. 2. During electrical vagal stimulation or feeding in (a) anaesthetized cats with ligated kidney vessels and resected jejunum and ileum, (b) anaesthetized normal cats or (c) conscious normal cats, release of the large molecular forms of gastrin, components I and II, was not detectable. 3. Luminal perfusates of cat antrum contained only component III and occasionally less than 2% of component IV. 4. Intravenous injections into conscious cats of components I, II and III isolated from cat antrum revealed a significantly slower elimination of the large components (t1/2 approximately 4.2 and 4.0 min respectively) than of component III (t1/2 approximately 1.1 min). Thus the absence of components I and II in cat blood cannot be due to rapid degradation. 5. During feeding, components I and II constituted 20% of the total gastrin immunoreactivity in antral venous blood of dogs. This amount is sufficient to account for the predominance of large components in peripheral venous blood in dogs considering their slow metabolic clearance rates. 6. Luminal perfusates of dog antrum contained components I, II and III in proportions corresponding to those found in antrum. 7. The results indicate that components I and II are synthesized in the same proportions in gastrin cells of cat and dog. In the cat, components I and II are, however, never released from the cells. Since these components contain heptadecapeptide gastrin within their sequeaces, they probably represent biosynthetic precursors of the principal gastrin, the heptadecapeptide (component III). The predominance of component III in gastric juice suggests that the feline gastrin cell secretes only component III, which degrades to component IV in blood.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Nilsson G. Appearance of gastrin in perfusates from the isolated gastric antrum of dogs. Scand J Gastroenterol. 1974;9(7):619–621. [PubMed] [Google Scholar]
- Becker H. D., Reeder D. D., Thompson J. C. Extraction of circulating endogenous gastrin by the small bowel. Gastroenterology. 1973 Dec;65(6):903–906. [PubMed] [Google Scholar]
- Benson D. F., LeMay M., Patten D. H., Rubens A. B. Diagnosis of normal-pressure hydrocephalus. N Engl J Med. 1970 Sep 17;283(12):609–615. doi: 10.1056/NEJM197009172831201. [DOI] [PubMed] [Google Scholar]
- Blair E. L., Grund E. R., Lund P. K., Piercy A., Reed J. D., Sanders D. J., Shale D., Shaw B., Wilkinson J. Comparison of vagal and meat stimulation on gastric acid secretion and serum gastrin. J Physiol. 1977 Mar;266(1):157–172. doi: 10.1113/jphysiol.1977.sp011761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair E. L., Grund E. R., Lund P. K., Sanders D. J. A possible origin of circulating gastrin component IV in cats. J Physiol. 1977 Dec;273(3):561–572. doi: 10.1113/jphysiol.1977.sp012110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniface J., Picone D., Schebalin M., Zfass A. M., Makhlouf G. M. Clearance rate, half-life, and secretory potency of human gastrin-17-I in different species. Gastroenterology. 1976 Aug;71(2):291–294. [PubMed] [Google Scholar]
- Booth R. A., Reeder D. D., Hjelmquist U. B., Brandt E. N., Jr, Thompson J. C. Renal inactivation of endogenous gastrin in dogs. Arch Surg. 1973 Jun;106(6):851–854. doi: 10.1001/archsurg.1973.01350180085024. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J., Debas H. T., Walsh J. H., Grossman M. I. Molecular forms of gastrin in antral mucosa and serum of dogs. Proc Soc Exp Biol Med. 1975 Jun;149(2):550–553. doi: 10.3181/00379727-149-38848. [DOI] [PubMed] [Google Scholar]
- GREGORY R. A., TRACY H. J. THE CONSTITUTION AND PROPERTIES OF TWO GASTRINS EXTRACTED FROM HOG ANTRAL MUCOSA. Gut. 1964 Apr;5:103–114. [PMC free article] [PubMed] [Google Scholar]
- Gregory R. A., Tracy H. J. Isolation of two "big gastrins" from Zollinger-Ellison tumour tissue. Lancet. 1972 Oct 14;2(7781):797–799. doi: 10.1016/s0140-6736(72)92151-4. [DOI] [PubMed] [Google Scholar]
- Gregory R. A., Tracy H. J. Isolation of two minigastrins from Zollinger-Ellison tumour tissue. Gut. 1974 Sep;15(9):683–685. doi: 10.1136/gut.15.9.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone. Evidence for an early biosynthetic precursor of proparathyroid hormone. J Biol Chem. 1976 Jul 10;251(13):3893–3899. [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F., Sundler F., Håkanson R. Pancreatic gastrin in foetal and neonatal rats. Nature. 1976 Aug 12;262(5569):609–610. doi: 10.1038/262609a0. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Stadil F. "Big gastrins" in the Zollinger-Ellison syndrome. Lancet. 1972 Dec 2;2(7788):1200–1200. doi: 10.1016/s0140-6736(72)92626-8. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Stadil F. Radioimmunoassay for gastrin employing immunosorbent. Scand J Clin Lab Invest. 1973 Jun;31(4):459–464. doi: 10.3109/00365517309084331. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Stadil F., Rubin B. Production and evaluation of antibodies for the radioimmunoassay of gastrin. Scand J Clin Lab Invest. 1972 Oct;30(2):221–232. doi: 10.3109/00365517209081114. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Stadil F., Vikelsoe J. Immunoreactive gastrin components in human serum. Gut. 1974 Feb;15(2):102–111. doi: 10.1136/gut.15.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadil F., Rehfeld J. F. Preparation of 125 I-labelled synthetic human gastrin I for radioimmunoanalysis. Scand J Clin Lab Invest. 1972 Dec;30(4):361–368. doi: 10.3109/00365517209080271. [DOI] [PubMed] [Google Scholar]
- Straus E., Yalow R. S. Studies on the distribution and degradation of heptadecapeptide, big, and big big gastrin. Gastroenterology. 1974 May;66(5):936–943. [PubMed] [Google Scholar]
- Uvnäs B., Uvnäs-Wallensten K., Nilsson G. Release of gastrin on vagal stimulation in the cat. Acta Physiol Scand. 1975 Jun;94(2):167–176. doi: 10.1111/j.1748-1716.1975.tb05876.x. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Debas H. T., Grossman M. I. Pure human big gastrin. Immunochemical properties, disappearance half time, and acid-stimulating action in dogs. J Clin Invest. 1974 Aug;54(2):477–485. doi: 10.1172/JCI107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. And now, "big, big" gastrin. Biochem Biophys Res Commun. 1972 Jul 25;48(2):391–395. doi: 10.1016/s0006-291x(72)80063-9. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Further studies on the nature of immunoreactive gastrin in human plasma. Gastroenterology. 1971 Feb;60(2):203–214. [PubMed] [Google Scholar]


