Abstract
Vitamin D3 (1,25-dihydroxycholecalciferol) induced the phagocyte oxidative burst and intracellular killing of Salmonella enterica serovar Typhimurium in a phosphatidylinositol 3-kinase-dependent manner. The antimicrobial effect was more pronounced for Salmonella SodCI and SodCII mutants, confirming the role of the phagocyte oxidase in the vitamin D3 effect. The results for an in vitro system with human THP-1 cells correlate with in vivo virulence data for mice and show that both the SodCI and SodCII enzymes are required to protect against the oxidative burst.
Salmonella enterica serovar Typhimurium causes self-limiting gastroenteritis in immunocompetent adults and may also cause life-threatening extraintestinal infections (7). The phagocyte oxidative burst is a key host defense mechanism against S. enterica serovar Typhimurium (3, 12, 17). S. enterica serovar Typhimurium produces three catalases and three periplasmic Cu,Zn superoxide dismutase (SodC) enzymes that may limit susceptibility to toxic products of the oxidative burst (1, 5, 8, 14). However, an S. enterica serovar Typhimurium mutant lacking two catalases is not attenuated for virulence in vivo (1). In contrast, Salmonella SodCI and SodCII isoenzymes have been implicated in virulence (3, 5). SodCI is encoded by the Gifsy-2 prophage and is found in strains associated with nontyphoid Salmonella bacteremia (5, 6, 8), while SodCII is the Escherichia coli SodC homologue and is expressed in all strains that have been examined (5). SodCIII is encoded by the Fels-1 phage, but its role in pathogenesis has not been established since SodCIII cannot replace the virulence function of SodCI (9). Vitamin D3 (1,25-dihydroxycholecalciferol) has been shown to induce antimicrobial activity in monocytic cells (2, 15) against Mycobacterium tuberculosis, which is mediated by a phosphatidylinositol 3-kinase (PI 3-K)-dependent pathway leading to an enhanced oxidative burst (16). In the present study, we examined whether vitamin D3 similarly affects the intracellular survival of Salmonella and the extent to which individual SodC enzymes protect Salmonella from the phagocyte oxidase.
Wild-type S. enterica serovar Typhimurium strain 14028s or mutants derived from this strain (MF1006 sodCI::aph, MF1007 sodCII::pRR10 [ΔtrfA], and MF 1008 sodCI::aph sodCII::pRR10 [ΔtrfA]) and described by Fang et al. (5) were grown to the stationary phase in minimal essential medium. Bacteria were opsonized with 50% normal human serum for 20 min at 37°C and then added to 1 × 106 phorbol myristate acetate (PMA) (20 ng/ml)-differentiated THP-1 cells at a ratio of 10:1 in six-well cell culture plates. The plates were centrifuged at 300 × g for 5 min and then incubated at 37°C in the presence of 5% CO2 for 30 min. The medium was removed and replaced with fresh medium containing gentamicin (RPMI 1640, 10% fetal calf serum, 20 μg of gentamicin per ml). In experiments in which a PI 3-K inhibitor or vitamin D3 was used, the inhibitor (15 μM LY294002 [Ly] or 50 nM wortmannin [Wm]) was added to cells immediately after addition of gentamicin-containing medium, and vitamin D3 (100 nM) was added to cultures 30 min after addition of the inhibitor.
Intracellular bacteria were harvested either immediately after infection and treatment or after 2, 4, or 8 h. The culture supernatants were removed, and adherent cells from each of three wells were lysed in 0.9 ml of deoxycholate (DOC) lysis buffer (0.5% DOC in 0.9% saline). Culture supernatants were centrifuged at 16,000 × g for 5 min, and the pellets were lysed in 0.1 ml of DOC lysis buffer and combined with 0.9 ml of lysed adherent cells. Tenfold serial dilutions were prepared in 0.9% saline, and 20-μl aliquots were plated. The culture plates were air dried and incubated at room temperature overnight, and then CFU were counted with a dissecting microscope.
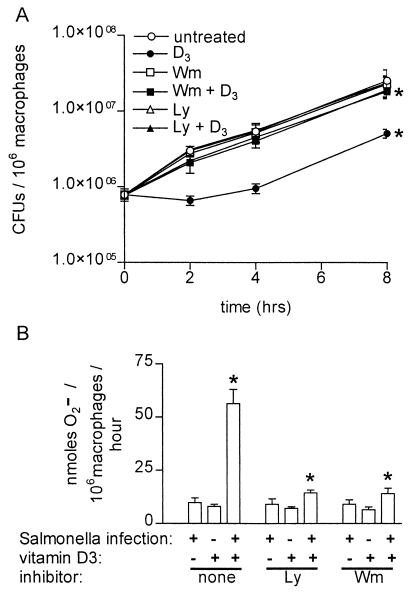
Vitamin D3 treatment had a pronounced, PI 3-K-dependent antimicrobial effect against wild-type S. enterica serovar Typhimurium in THP-1 cells, reducing the number of viable bacteria by almost 10-fold in 8 h compared to the number of bacteria in untreated cells (Fig. 1A ). Vitamin D3 had no effect on the extracellular growth of S. enterica serovar Typhimurium in culture (data not shown). Incubation of monocytes with Wm and Ly prior to addition of vitamin D3 restored the levels of bacterial growth to 81 and 76%, respectively, of the levels observed in untreated cells after 8 h of infection. Vitamin D3-induced killing of S. enterica serovar Typhimurium was not dependent on nitric oxide, as no nitrite was detectable by the Griess assay in culture supernatants from the cells (data not shown). Decreased intracellular growth of S. enterica serovar Typhimurium correlated with the amount of superoxide produced as measured by the Sod-inhibitable reduction of ferricytochrome c (4). Vitamin D3-activated, Salmonella-infected THP-1 cells produced a significantly larger amount of superoxide than cells exposed to either infection alone or to vitamin D3 alone (Fig. 1B). The amount of superoxide produced by vitamin D3-treated, Salmonella-infected THP-1 cells was reduced to background levels by pretreatment with either Ly or Wm (Fig. 1B). Interestingly, S. enterica serovar Typhimurium grew rapidly in PMA-differentiated THP-1 cells (Fig. 1) compared with the growth in primary human monocyte-derived macrophages, in which growth of intracellular Salmonella was reported to occur only in a subpopulation of cells (11).
FIG. 1.
Vitamin D3 restricts the intracellular growth of wild-type S. enterica serovar Typhimurium and induces the phagocyte oxidative burst in a PI 3-kinase-dependent manner. (A) Intracellular S. enterica serovar Typhimurium quantitated by serial dilution in Luria-Bertani broth and plating on Luria-Bertani agar 0, 2, 4, and 8 h after infection of THP-1 cells. Cells were either not treated or treated with the PI 3-K inhibitor Wm (50 nM) or Ly (15 μM) and then were left untreated or treated with vitamin D3 (100 nM). The data are averages from three independent experiments performed in triplicate. An asterisk indicates that the P value was <0.03 when the growth in vitamin D3-treated cells was compared to the growth in control cells or when the growth in cells treated with Ly or Wm plus vitamin D3 was compared to the growth in cells treated only with vitamin D3, as determined by analysis of variance at each time point. (B) Superoxide secreted by macrophages in 1 h determined by measuring the Sod-inhibitable reduction of ferricytochrome c in the presence and absence of Salmonella infection, vitamin D3 treatment, and the inhibitor Ly or Wm. An asterisk indicates that the P value was <0.02 when the data for Salmonella infection and vitamin D3 treatment was compared to the data for either treatment alone or when the data for pretreatment with Ly or Wm followed by Salmonella infection and vitamin D3 treatment was compared to the data for no pretreatment, as determined by an unpaired Student's t test.
Control of S. enterica serovar Typhimurium infection in the mouse model requires NADPH oxidase activity (3, 6, 12). The contribution of the phagocyte oxidase is evident after 5 days of infection in the mouse model (12) and is also seen in in vitro-activated peritoneal macrophages. In these cells, anti-Salmonella activity was observed within several hours after infection (17), which is similar to the findings in the present study obtained with vitamin D3-activated THP-1 cells. SodCI is required for full virulence in mice; the effect is more pronounced in C3H/HeN mice (Ityr), and this has been shown to involve protection from both NADPH oxidase and nitric oxide synthase activities (3, 5, 6). The SodCI mutant also shows decreased survival in activated, but not resting, murine macrophages (3, 6). A mutation in SodCII also attenuates infectivity in C3H/HeN mice, and the virulence of the double SodCI-SodCII mutant is less than the virulence of either of the single Sod knockouts (5). The role of SodCII in intramacrophage survival has not been reported.
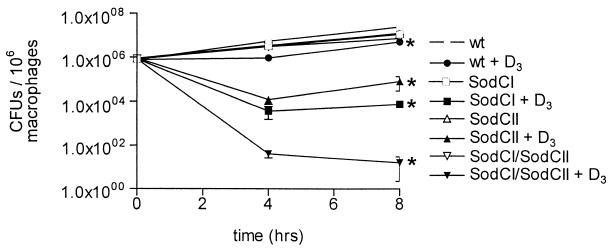
In the present study, SodC mutants in THP-1 cells were more susceptible to the effect of vitamin D3 than wild-type organisms were. While each mutant strain (SodCI, SodCII, and the SodCI-SodCII double knockout) grew normally in untreated THP-1 cells, considerable loss of viability was observed in vitamin D3-treated cells (Fig. 2). Eight hours after infection, the numbers of CFU of SodCI and SodCII mutants recovered in THP-1 cells treated with vitamin D3 were 0.05 and 0.66%, respectively, of the number of CFU recovered from untreated THP-1 cells. Moreover, the SodCI-SodCII mutant was almost completely killed in vitamin D3-treated cells at 4 h after infection. These results show that both SodCI and SodCII contribute to the ability of S. enterica serovar Typhimurium to survive the oxidative burst.
FIG. 2.
S. enterica serovar Typhimurium Sod mutants are more susceptible to the antimicrobial activity of vitamin D3 than the wild-type strain. Differentiated THP-1 cells were infected with wild-type S. enterica serovar Typhimurium (wt) or with a SodCI, SodCII, or double SodCI-SodCII S. enterica serovar Typhimurium mutant and then were left untreated or were treated with vitamin D3. The CFU of intracellular bacteria were enumerated 0, 4, and 8 h after infection. The data are averages from three independent experiments performed in triplicate. An asterisk indicates that the P value was <0.05 when the growth of each mutant in vitamin D3-treated cells was compared to the growth in control cells, as determined by analysis of variance at each time point.
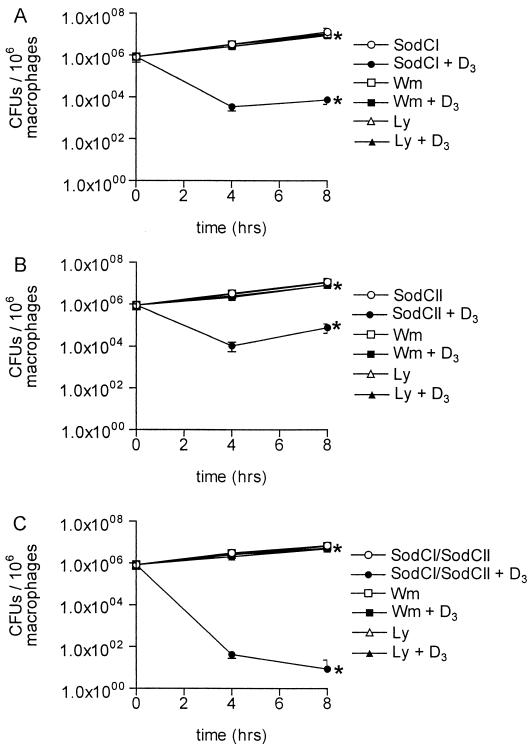
Both PI 3-K inhibitors reduced the killing of mutant S. enterica serovar Typhimurium strains in vitamin D3-treated THP-1 cells. Growth of the SodCI mutant was restored to more than 75% of the growth observed in control cells by 8 h after infection (Fig. 3A). Growth of the SodCII mutant was restored to more than 68% of the growth observed in control cells by 8 h after infection (Fig. 3B). Finally, growth of the SodCI-SodCII double mutant was restored to more than 70% of the control levels by 8 h after infection (Fig. 3C).
FIG. 3.
Killing of intracellular S. enterica serovar Typhimurium Sod mutants by vitamin D3 is PI 3-K dependent. Differentiated THP-1 cells were infected with the SodCI mutant (A), the SodCII mutant (B), and the SodCI-SodCII double mutant (C). Cells either were left untreated or were treated with Wm or Ly and then were either left untreated or treated with vitamin D3. The CFU of intracellular bacteria were enumerated 0, 4, and 8 h after infection. The data are from three independent experiments performed in triplicate. An asterisk indicates that the P value was <0.04 (A), <0.05 (B), or <0.01 (C) when growth in vitamin D3-treated cells was compared to growth in control cells or when the data for pretreatment with Ly or Wm followed by Salmonella infection and vitamin D3 treatment of cells was compared to the data for no pretreatment of cells. Comparisons were made by performing analysis of variance at each time point.
Dependence of vitamin D3-mediated anti-Salmonella activity on PI 3-K and the oxidative burst is consistent with a previous report which showed that PI 3-K is required for vitamin D3-induced, oxidase-mediated inhibition of the intracellular survival of M. tuberculosis in PMA-differentiated THP-1 cells and primary human monocytes (16). This system was shown to be independent of nitric oxide (16). Thus, the present data provide evidence that the SodC enzymes are required for survival in the presence of reactive oxygen intermediates during infection of human cells that do not express nitric oxide synthase activity. Periplasmic Sod enzymes from other microorganisms have also been shown to play a role in protection from reactive oxygen species in the absence of nitric oxide production. An M. tuberculosis SodC mutant was shown to be more susceptible to killing by exogenously added H2O2 and by gamma interferon-activated murine peritoneal macrophages which produce abundant amounts of superoxide. In contrast, this SodC mutant was not more susceptible to killing by either resting macrophages or activated macrophages derived from oxidative burst-deficient mice (13). E. coli SodC mutants were also shown to be more susceptible to killing by exogenous H2O2 during early-stationary-phase growth than their wild-type counterparts (10).
The results obtained here with THP-1 cells contrast with earlier findings obtained with murine macrophages, in which the survival of sodCI mutants of S. enterica serovar Typhimurium, S. enterica serovar Dublin, and S. enterica serovar Choleraesuis in nonactivated peritoneal macrophages from Itys mice was no different than the survival of wild-type strains (6). In contrast, the findings of the present study, in which vitamin D3-activated human cells were used, are congruent with a report that a sodCI mutant of S. enterica serovar Typhimurium was killed more efficiently than wild type bacilli by gamma interferon-activated murine macrophages (3). However, in the latter study, decreased survival was dependent on a combination of nitric oxide synthase and oxidase activity.
In summary, our results show that vitamin D3 induces bactericidal activity against intracellular S. enterica serovar Typhimurium in human monocyte-like cells that is PI 3-K dependent and mediated by the phagocyte oxidase. Compared to the bactericidal effect of individual SodC mutants, the bactericidal effect is more pronounced with the SodCI-SodCII double mutant, indicating that both Sod enzymes contribute to bacterial resistance to oxidative damage. In addition, the Salmonella Sod enzymes provide protection against the phagocyte oxidase products in the absence of nitric oxide production. These data provide additional in vitro evidence that supports a protective role for S. enterica serovar Typhimurium SodCI and SodCII during intracellular infection.
Acknowledgments
This work was supported by Canadian Institutes for Health Research grant MOP-8633 (to N.E.R.) and by U.S. Public Health Service grants AI32178 and DK35108 (to D.G.G.).
We thank Ferric C. Fang for kindly supplying the sodC mutant strains.
Editor: D. L. Burns
REFERENCES
- 1.Buchmeier, N. A., S. J. Libby, Y. Xu, P. C. Loewen, J. Switala, D. G. Guiney, and F. C. Fang. 1995. DNA repair is more important than catalase for Salmonella virulence in mice. J. Clin. Investig. 95:1047-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowle, A. J., E. J. Ross, and M. H. May. 1987. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect. Immun. 55:2945-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Groote, M. A., U. A. Ochsner, M. U. Shiloh, C. Nathan, J. M. McCord, M. C. Dinauer, S. J. Libby, A. Vazquez-Torres, Y. Xu, and F. C. Fang. 1997. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. USA 94:13997-14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLeo, F. R., M. A. Jutila, and M. T. Quinn. 1996. Characterization of peptide diffusion into electropermeabilized neutrophils. J. Immunol. Methods 198:35-49. [DOI] [PubMed] [Google Scholar]
- 5.Fang, F. C., M. A. DeGroote, J. W. Foster, A. J. Baumler, U. Ochsner, T. Testerman, S. Bearson, J. C. Giard, Y. Xu, G. Campbell, and T. Laessig. 1999. Virulent Salmonella typhimurium has two periplasmic Cu, Zn-superoxide dismutases. Proc. Natl. Acad. Sci. USA 96:7502-7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrant, J. L., A. Sansone, J. R. Canvin, M. J. Pallen, P. R. Langford, T. S. Wallis, G. Dougan, and J. S. Kroll. 1997. Bacterial copper- and zinc-cofactored superoxide dismutase contributes to the pathogenesis of systemic salmonellosis. Mol. Microbiol. 25:785-796. [DOI] [PubMed] [Google Scholar]
- 7.Fierer, J., and D. G. Guiney. 2001. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J. Clin. Investig. 107:775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueroa-Bossi, N., and L. Bossi. 1999. Inducible prophages contribute to Salmonella virulence in mice. Mol. Microbiol. 33:167-176. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa-Bossi, N., S. Uzzau, D. Maloriol, and L. Bossi. 2001. Variable assortment of prophages provides a transferable repertoire of pathogenic determinants in Salmonella. Mol. Microbiol. 39:260-271. [DOI] [PubMed] [Google Scholar]
- 10.Gort, A. S., D. M. Ferber, and J. A. Imlay. 1999. The regulation and role of the periplasmic copper, zinc superoxide dismutase of Escherichia coli. Mol. Microbiol. 32:179-191. [DOI] [PubMed] [Google Scholar]
- 11.Libby, S. J., M. Lesnick, P. Hasegawa, E. Weidenhammer, and D. G. Guiney. 2000. The Salmonella virulence plasmid spv genes are required for cytopathology in human monocyte-derived macrophages. Cell Microbiol. 2:49-58. [DOI] [PubMed] [Google Scholar]
- 12.Mastroeni, P., A. Vazquez-Torres, F. C. Fang, Y. Xu, S. Khan, C. E. Hormaeche, and G. Dougan. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 192:237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piddington, D. L., F. C. Fang, T. Laessig, A. M. Cooper, I. M. Orme, and N. A. Buchmeier. 2001. Cu,Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect. Immun. 69:4980-4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbe-Saule, V., C. Coynault, M. Ibanez-Ruiz, D. Hermant, and F. Norel. 2001. Identification of a non-haem catalase in Salmonella and its regulation by RpoS (sigma S). Mol. Microbiol. 39:1533-1545. [DOI] [PubMed] [Google Scholar]
- 15.Rook, G. A., J. Steele, L. Fraher, S. Barker, R. Karmali, J. O'Riordan, and J. Stanford. 1986. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology 57:159-163. [PMC free article] [PubMed] [Google Scholar]
- 16.Sly, L. M., M. Lopez, W. M. Nauseef, and N. E. Reiner. 2001. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J. Biol. Chem. 276:35482-35493. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez-Torres, A., J. Jones-Carson, P. Mastroeni, H. Ischiropoulos, and F. C. Fang. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]