Abstract
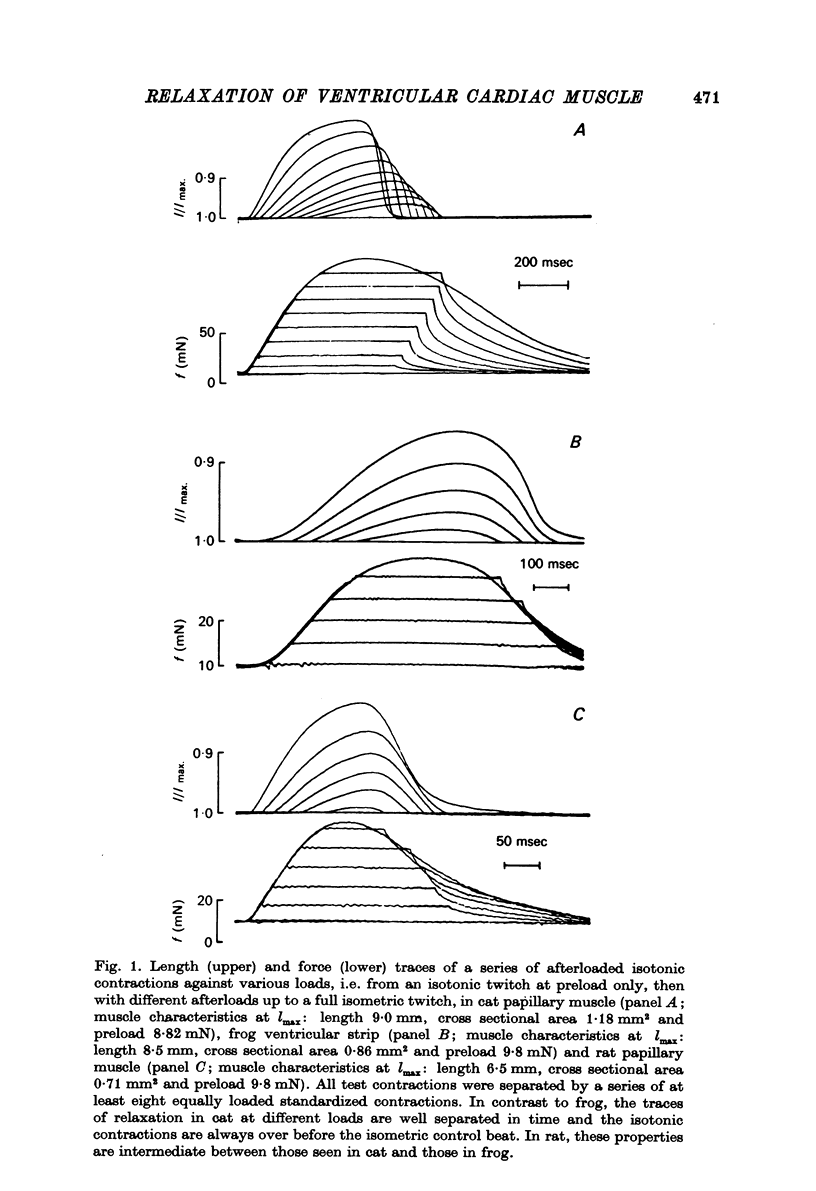
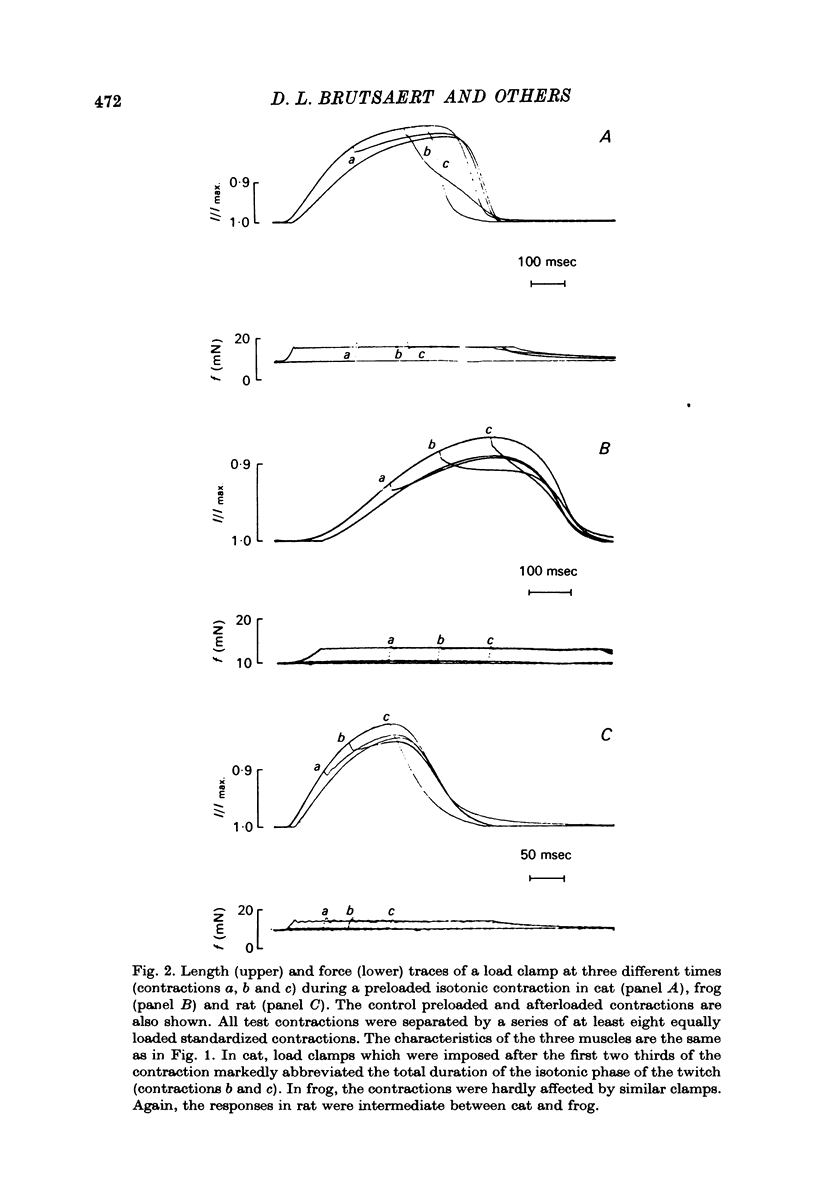
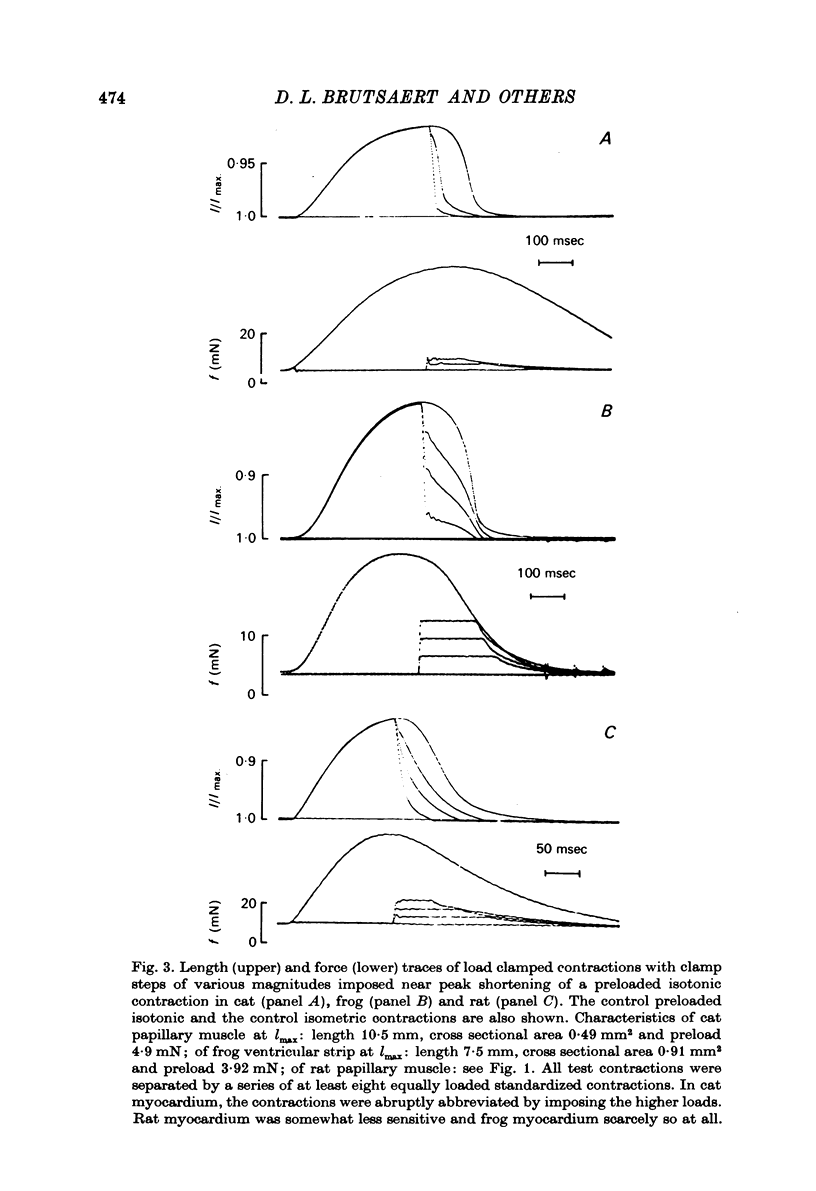
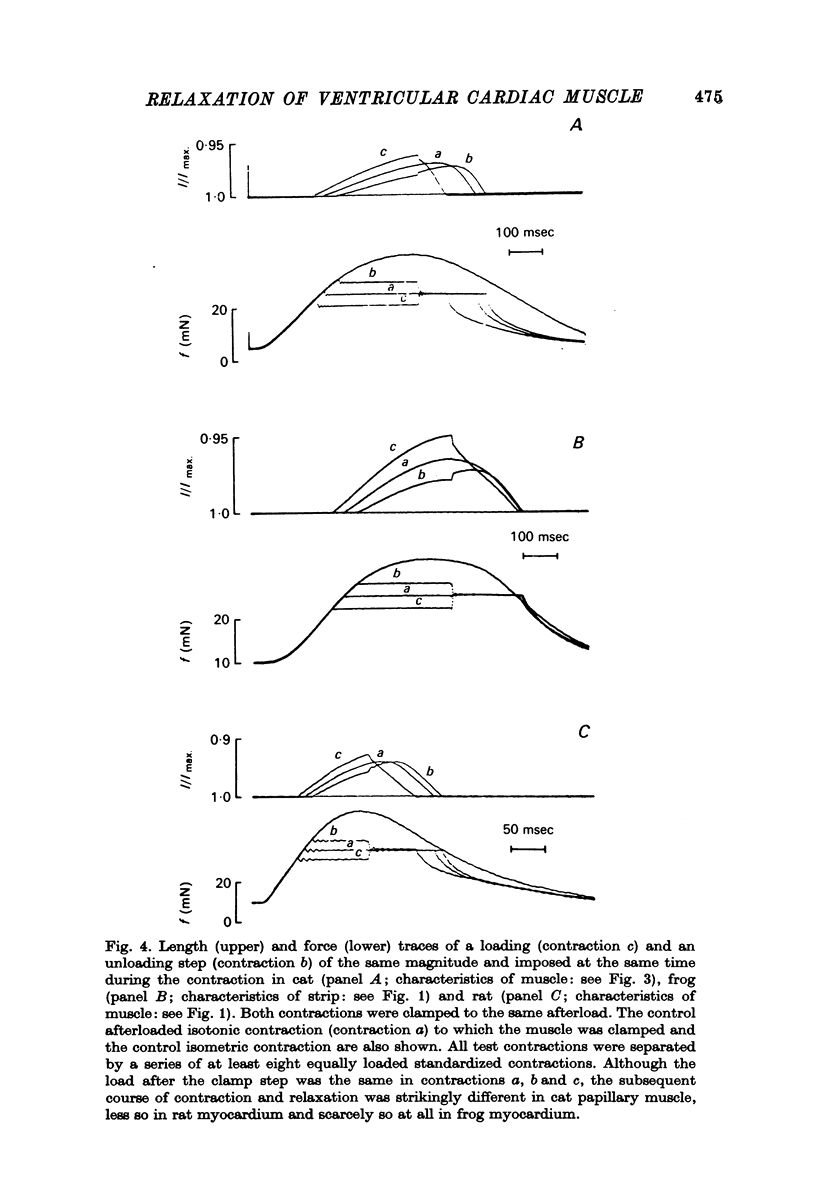
1. The load bearing capacity during relaxation of ventricular cardiac muscle from various animal species was investigated. 2. The effect of load on the time course of relaxation was analysed either by comparing afterloaded contractions against various loads or by imposing abrupt alterations in load (load clamps). 3. In heart muscle from the mammalian species studied relaxation was sensitive to loading conditions, whereas in frog heart muscle relaxation was largely independent of the loading conditions. The mechanical properties of relaxation of cardiac muscle appear, therefore, governed by the interplay of a load-controlled and an activation-controlled decay mechanism, the relative importance of which differs with species. 4. Load-dependence may be the mechanical expression of the ratio of the number of force generating sites at any time during contraction and relaxation to the load to be carried; this mechanism would predominate in mammalian animal species with a well developed calcium sequestering sarcoplasmic reticulum. Activation-dependence would seem to predominate in animal species, such as frog, in which calcium sequestration appears to be the rate limiting step during relaxation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady A. J. Length-tension relations in cardiac muscle. Am Zool. 1967 Aug;7(3):603–610. doi: 10.1093/icb/7.3.603. [DOI] [PubMed] [Google Scholar]
- Briggs F. N., Poland J. L., Solaro R. J. Relative capabilities of sarcoplasmic reticulum in fast and slow mammalian skeletal muscles. J Physiol. 1977 Apr;266(3):587–594. doi: 10.1113/jphysiol.1977.sp011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert D. L., Claes V. A., De Clerck N. M. Relaxation of mammalian single cardiac cells after pretreatment with the detergent Brij-58. J Physiol. 1978 Oct;283:481–491. doi: 10.1113/jphysiol.1978.sp012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert D. L., Claes V. A., Donders J. J. Effects of controlling the velocity of shortening on force-velocity-length and time relations in cat papillary muscle. Velocity clamping. Circ Res. 1972 Mar;30(3):310–315. doi: 10.1161/01.res.30.3.310. [DOI] [PubMed] [Google Scholar]
- Brutsaert D. L., Claes V. A. Onset of mechanical activation of mammalian heart muscle in calcium- and strontium-containing solutions. Circ Res. 1974 Sep;35(3):345–357. doi: 10.1161/01.res.35.3.345. [DOI] [PubMed] [Google Scholar]
- Brutsaert D. L., Housmans P. R. Load clamp analysis of maximal force potential of mammalian cardiac muscle. J Physiol. 1977 Oct;271(3):587–603. doi: 10.1113/jphysiol.1977.sp012016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert D. L., Paulus W. J. Loading and performance of the heart as muscle and pump. Cardiovasc Res. 1977 Jan;11(1):1–16. doi: 10.1093/cvr/11.1.1. [DOI] [PubMed] [Google Scholar]
- Chapman R. A. The onic dependence of the strength and spontaneous relations of the potassium contracture induced in the heart of the frog Rana pipiens. J Physiol. 1973 Jun;231(2):209–232. doi: 10.1113/jphysiol.1973.sp010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes V. A., Brutsaert D. L. Infrared-emitting diode and optic fibers for underwater force measurement in heart muscle. J Appl Physiol. 1971 Sep;31(3):497–498. doi: 10.1152/jappl.1971.31.3.497. [DOI] [PubMed] [Google Scholar]
- Cleworth D. R., Edman K. A. Changes in sarcomere length during isometric tension development in frog skeletal muscle. J Physiol. 1972 Dec;227(1):1–17. doi: 10.1113/jphysiol.1972.sp010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Nilsson E. Time course of the active state in relation to muscle length and movement: a comparative study on skeletal muscle and myocardium. Cardiovasc Res. 1971 Jul;Suppl 1:3–10. doi: 10.1093/cvr/5.supp1.3. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium release from the sarcoplasmic reticulum. Circ Res. 1977 Feb;40(2):119–129. doi: 10.1161/01.res.40.2.119. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A. Excitation-contraction coupling and digitalis. Circulation. 1973 Jan;47(1):5–7. doi: 10.1161/01.cir.47.1.5. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M., Kimoto Y., Saito M., Wada Y. Tension fall after contraction of bullfrog atrial muscle examined with the voltage clamp technique. Jpn J Physiol. 1972 Dec;22(6):637–650. doi: 10.2170/jjphysiol.22.637. [DOI] [PubMed] [Google Scholar]
- Henderson A. H., Brutsaert D. L. Force-velocity-length relationship in heart muscle: lack of time-independence during twitch contractions of frog ventricle strips with caffeine. Pflugers Arch. 1974 Apr 4;348(1):59–64. doi: 10.1007/BF00587739. [DOI] [PubMed] [Google Scholar]
- Henderson A. H., Brutsaert D. L., Parmley W. W., Sonnenblick E. H. Myocardial mechanics in ppillary muscles of the rat and cat. Am J Physiol. 1969 Nov;217(5):1273–1279. doi: 10.1152/ajplegacy.1969.217.5.1273. [DOI] [PubMed] [Google Scholar]
- Hill D. K. Resting tension and the form of the twitch of rat skeletal muscle at low temperature. J Physiol. 1972 Feb;221(1):161–171. doi: 10.1113/jphysiol.1972.sp009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housmans P. R., Brutsaert D. L. Three-step yielding of load-clamped mammalian cardiac muscle. Nature. 1976 Jul 1;262(5563):56–58. doi: 10.1038/262056a0. [DOI] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. The mechanical properties of relaxing muscle. J Physiol. 1960 Jun;152:30–47. doi: 10.1113/jphysiol.1960.sp006467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell B. R. A reexamination of the influence of muscle length on myocardial performance. Circ Res. 1977 Mar;40(3):221–230. doi: 10.1161/01.res.40.3.221. [DOI] [PubMed] [Google Scholar]
- Jewell B. R., Rovell J. M. Influence of previous mechanical events on the contractility of isolated cat papillary muscle. J Physiol. 1973 Dec;235(3):715–740. doi: 10.1113/jphysiol.1973.sp010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L. The concept of active state in striated muscle. Circ Res. 1976 Feb;38(2):53–59. doi: 10.1161/01.res.38.2.53. [DOI] [PubMed] [Google Scholar]
- KELLY J. J., Jr, HOFFMAN B. F. Mechanical activity of rat papillary muscle. Am J Physiol. 1960 Jul;199:157–162. doi: 10.1152/ajplegacy.1960.199.1.157. [DOI] [PubMed] [Google Scholar]
- Katz A. M. Contractile proteins of the heart. Physiol Rev. 1970 Jan;50(1):63–158. doi: 10.1152/physrev.1970.50.1.63. [DOI] [PubMed] [Google Scholar]
- Kaufmann R. L., Lab M. J., Hennekes R., Krause H. Feedback interaction of mechanical and electrical events in the isolated mammalian ventricular myocardium (cat papillary muscle). Pflugers Arch. 1971;324(2):100–123. doi: 10.1007/BF00592656. [DOI] [PubMed] [Google Scholar]
- Mainwood G. W., McGuigan J. A. Evidence for inward calcium current in the absence of external sodium in rat myocardium. Experientia. 1975 Jan 15;31(1):67–69. doi: 10.1007/BF01924683. [DOI] [PubMed] [Google Scholar]
- Meerson F. Z., Kapelko V. I. The significance of the interrelationship between the intensity of the contractile state and the velocity of relaxation in adapting cardiac muscle to function at high work loads. J Mol Cell Cardiol. 1975 Nov;7(11):793–806. doi: 10.1016/0022-2828(75)90131-5. [DOI] [PubMed] [Google Scholar]
- Morad M., Rolett E. L. Relaxing effects of catecholamines on mammalian heart. J Physiol. 1972 Aug;224(3):537–558. doi: 10.1113/jphysiol.1972.sp009912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. Movements of Ca in beating ventricles of the frog heart. J Physiol. 1963 Jul;167:551–580. doi: 10.1113/jphysiol.1963.sp007167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley W. W., Brutsaert D. L., Sonnenblick E. H. Effects of altered loading on contractile events in isolated cat papillary muscle. Circ Res. 1969 Apr;24(4):521–532. doi: 10.1161/01.res.24.4.521. [DOI] [PubMed] [Google Scholar]
- Parmley W. W., Sonnenblick E. H. Relation between mechanics of contraction and relaxation in mammalian cardiac muscle. Am J Physiol. 1969 May;216(5):1084–1091. doi: 10.1152/ajplegacy.1969.216.5.1084. [DOI] [PubMed] [Google Scholar]
- Parsons C., Porter K. R. Muscle relaxation: evidence for an intrafibrillar restoring force in vertebrate striated muscle. Science. 1966 Jul 22;153(3734):426–427. doi: 10.1126/science.153.3734.426. [DOI] [PubMed] [Google Scholar]
- Sandow A. Skeletal muscle. Annu Rev Physiol. 1970;32:87–138. doi: 10.1146/annurev.ph.32.030170.000511. [DOI] [PubMed] [Google Scholar]
- Staley N. A., Benson E. S. The ultrastructure of frog ventricular cardiac muscle and its relationship to mechanism of excitation-contraction coupling. J Cell Biol. 1968 Jul;38(1):99–114. doi: 10.1083/jcb.38.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauer B. E. Force-velocity relations of isotonic relaxation in mammalian heart muscle. Am J Physiol. 1973 Feb;224(2):431–434. doi: 10.1152/ajplegacy.1973.224.2.431. [DOI] [PubMed] [Google Scholar]
- Strobeck J. E., Bahler A. S., Sonnenblick E. H. Isotonic relaxation in cardiac muscle. Am J Physiol. 1975 Sep;229(3):646–651. doi: 10.1152/ajplegacy.1975.229.3.646. [DOI] [PubMed] [Google Scholar]
- Suga H., Yamakoshi K. I. Reduction of the duration of isovolumic relaxation in the ejecting left ventricle of the dog: residual volume clamping. J Physiol. 1977 May;267(1):63–74. doi: 10.1113/jphysiol.1977.sp011801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya K., Kikkawa S., Gunji A., Hori M., Sakurai Y. Maximum rate of tension fall during isometric relaxation at end-systolic fiber length in canine papillary muscle. Circ Res. 1977 Jun;40(6):584–589. doi: 10.1161/01.res.40.6.584. [DOI] [PubMed] [Google Scholar]
- Vassort G. Influence of sodium ions on the regulation of frog myocardial contractility. Pflugers Arch. 1973 Mar 30;339(3):224–240. doi: 10.1007/BF00587374. [DOI] [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]
- Winegrad S. Intracellular calcium binding and release in frog heart. J Gen Physiol. 1973 Dec;62(6):693–706. doi: 10.1085/jgp.62.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]


