Abstract
1. In the hand area of the post-central gyrus of three alert Macaca speciosa monkeys neurones related to cutaneous receptors but not activated by simple touch on the receptive field were recorded using the transdural micro-electrode recording technique. Thirty-six cells were found to have complex cutaneous receptive field properties. These neurones were subdivided into the following three groups. 2. Nine neurones were not activated by punctate stimuli on the receptive fields but responded well to movement along the skin. The activity of these neurones was not affected by the direction of movement; nor was it sensitive to different textures of the moving surface. 3. Eighteen neurones responded to cutaneous movement along the skin surface in a particular direction giving no response to stimulation in the opposite direction and intermediate responses to intermediate directions. Similar responses were evoked from different subparts of the receptive field. 4. Nine neurones responded well to an edge placed on the skin in an optimal orientation or moved along the skin in a direction perpendicular to the edge. A maximal response was produced by stimuli of the same optimal orientation in different parts of the receptive field. The significance of the stimuli to the monkey had only a minor influence on the magnitude of the responses of these neurones and no influence on the receptive field properties. 5. The occurrence of the complex cutaneous cells increased from anterior to posterior within the post-central gyrus and most of them were found in Brodmann's area 2. Thus we postulate that the complex receptive field properties arise as a consequence of cortical processing in a network in which postsynaptic one-way lateral inhibition generates the directional properties of the neurones. 6. The complex cutaneous neurones constituted only 6% of the neurones studied in the hand area of the post-central gyrus. Thus the prevalence of neurones with elongated and direction-selective receptive fields is low in the primary somatosensory cortex in comparison with the visual cortex. These neurones may, however, serve the sterognostic capcity of the hand by contributing information about stimulus motion, orientation and direction of movement on the skin.
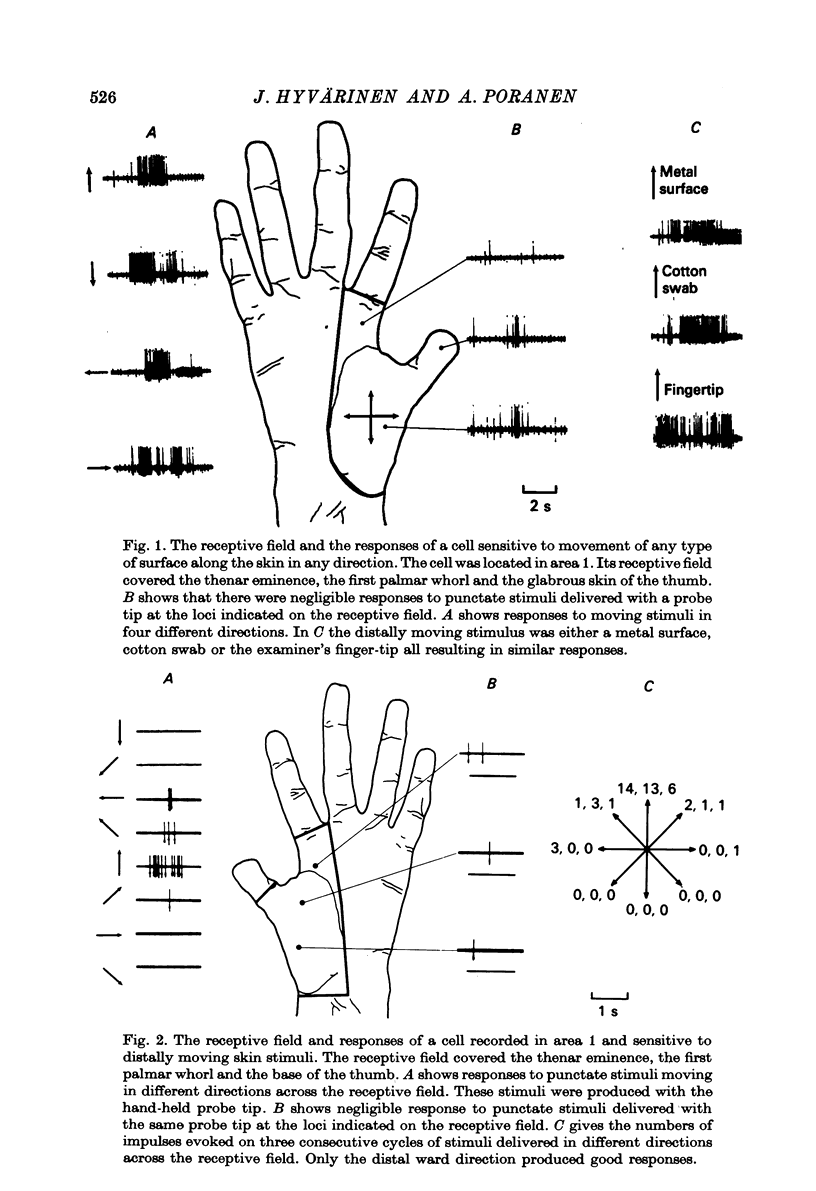
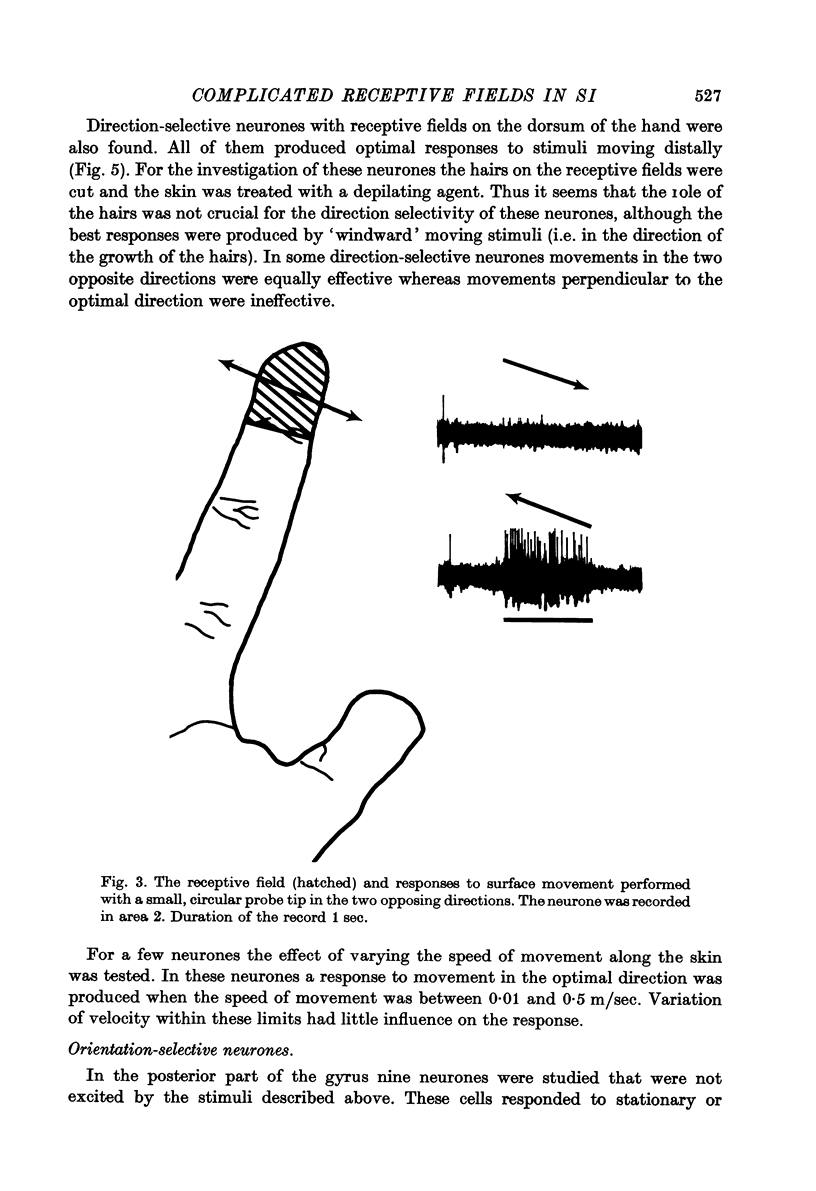
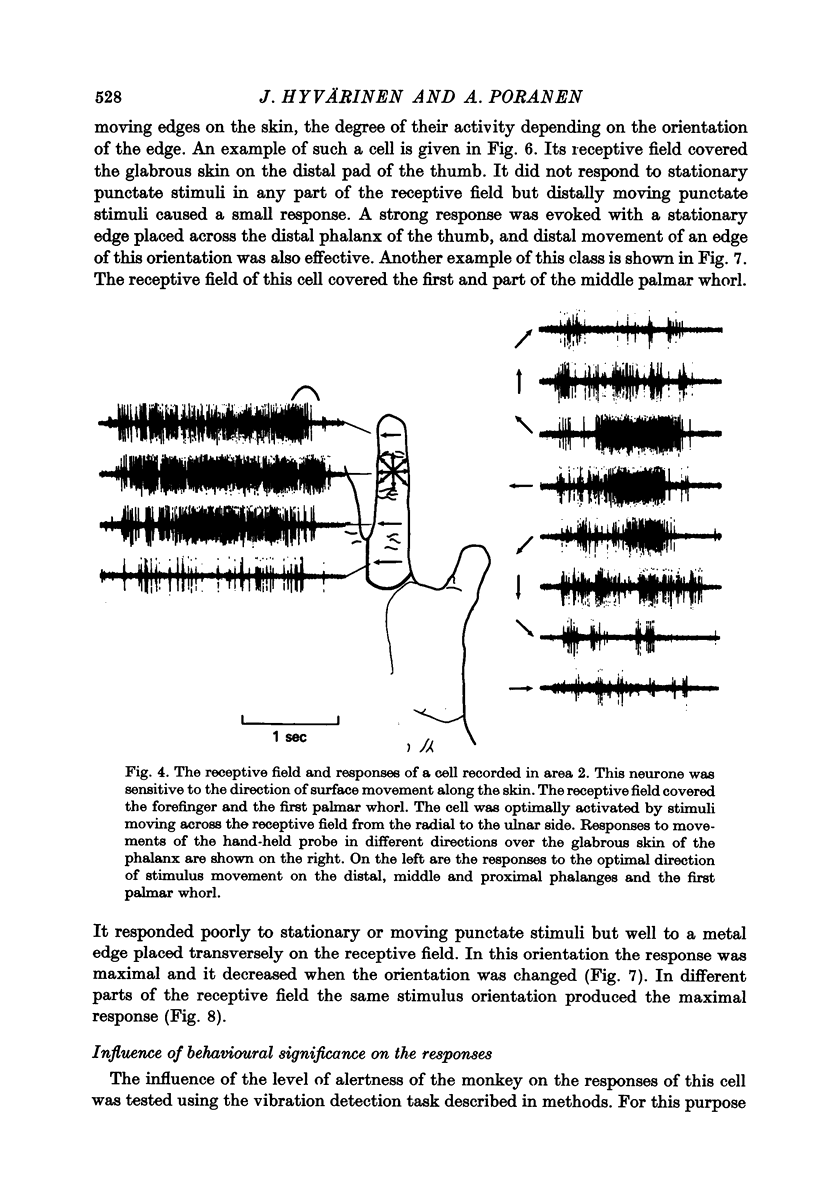
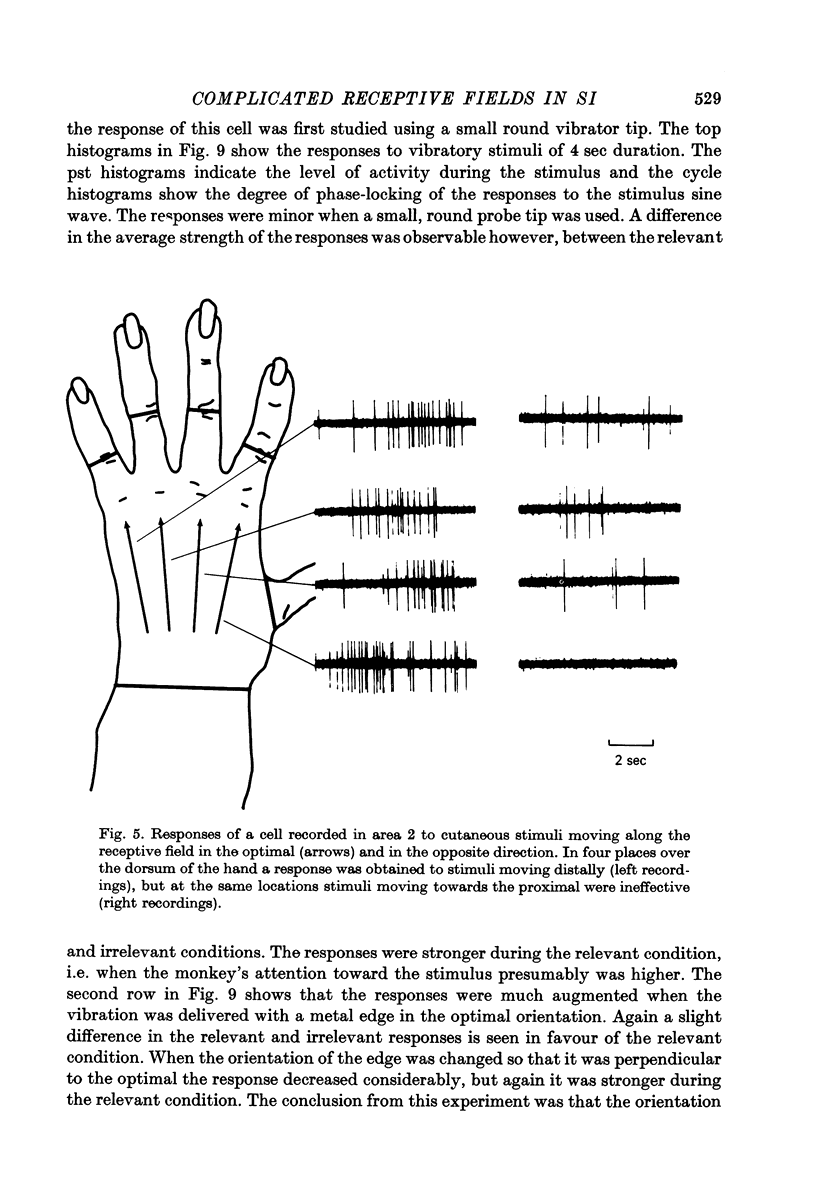
Full text
PDF


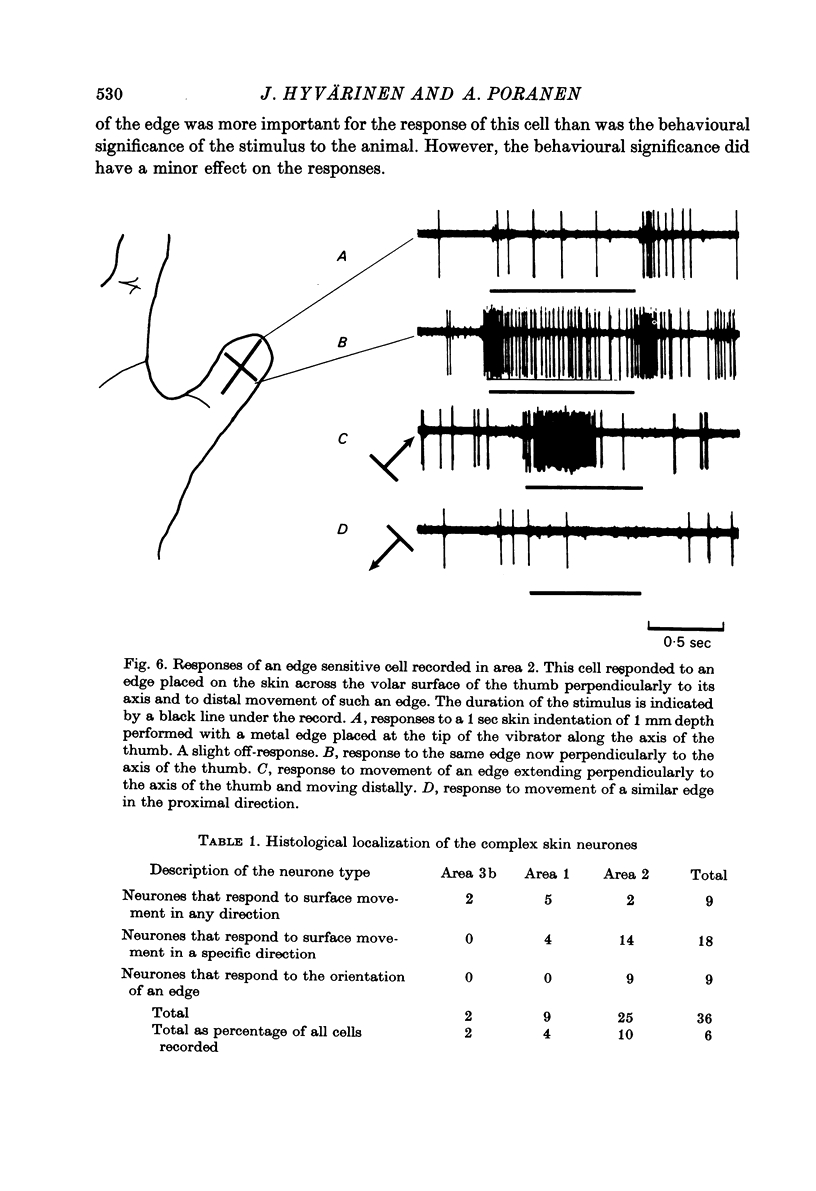
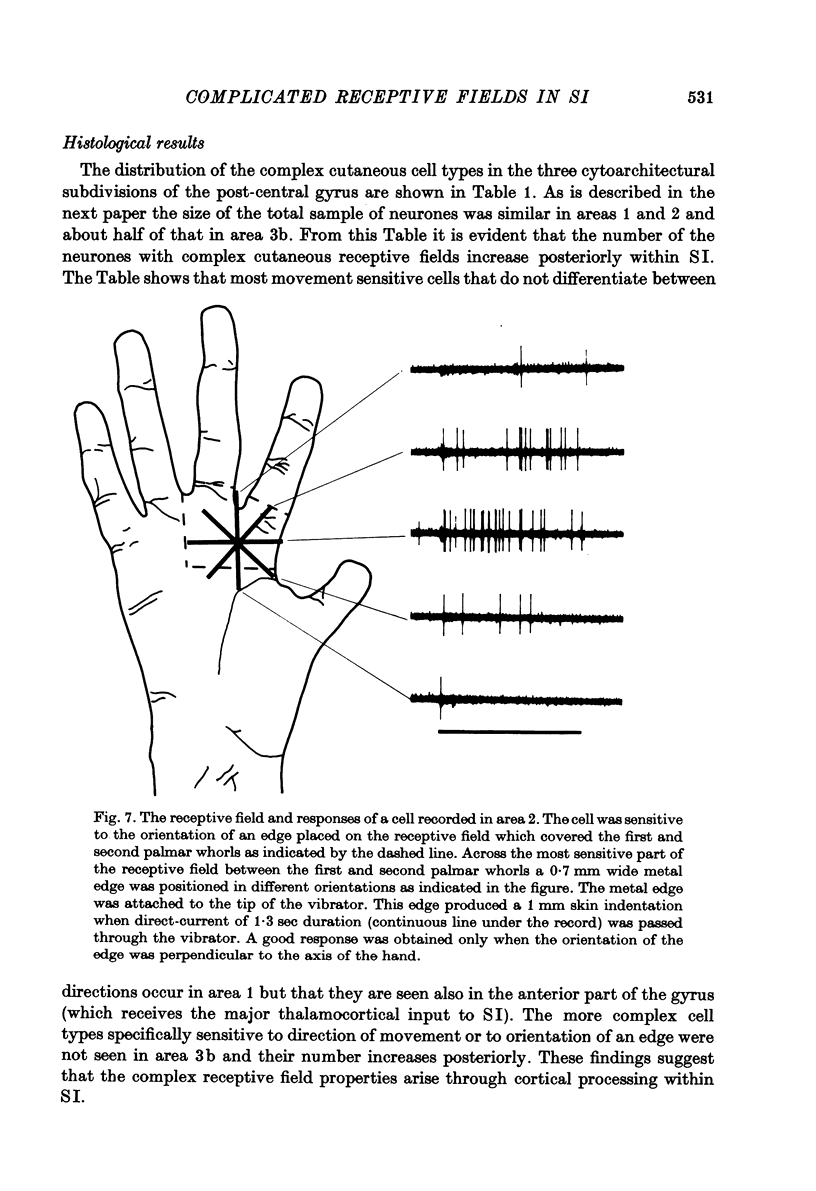
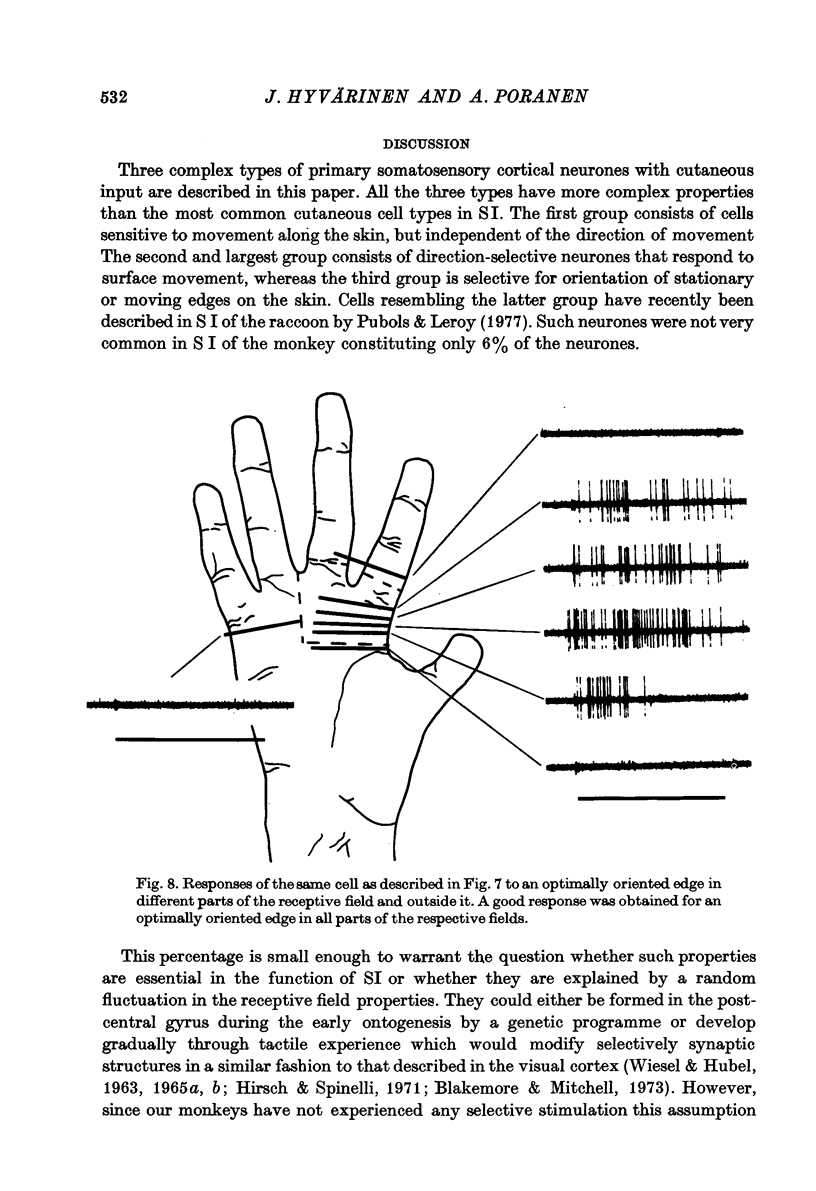
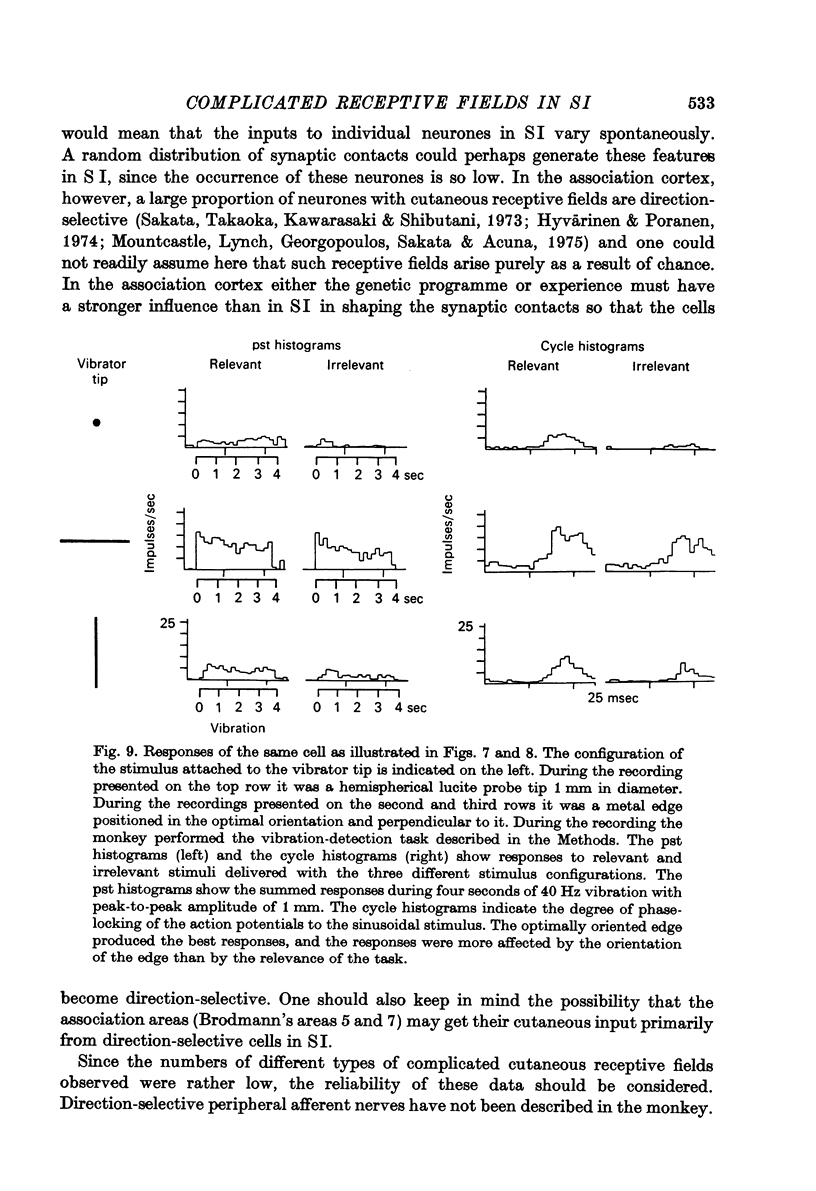



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Levick W. R. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965 Jun;178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Mitchell D. E. Environmental modification of the visual cortex and the neural basis of learning and memory. Nature. 1973 Feb 16;241(5390):467–468. doi: 10.1038/241467a0. [DOI] [PubMed] [Google Scholar]
- Evarts E. V. A technique for recording activity of subcortical neurons in moving animals. Electroencephalogr Clin Neurophysiol. 1968 Jan;24(1):83–86. doi: 10.1016/0013-4694(68)90070-9. [DOI] [PubMed] [Google Scholar]
- Fernald R. D. A neuron model with spatially distributed synaptic input. Biophys J. 1971 Apr;11(4):323–340. doi: 10.1016/S0006-3495(71)86218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald R. D., Gerstein G. L. Response of cat cochlear nucleus neurons to frequency and amplitude modulated tones. Brain Res. 1972 Oct 27;45(2):417–435. doi: 10.1016/0006-8993(72)90472-6. [DOI] [PubMed] [Google Scholar]
- Friendlich A. R. Primate head restrainer using a nonsurgical technique. J Appl Physiol. 1973 Dec;35(6):934–935. doi: 10.1152/jappl.1973.35.6.934. [DOI] [PubMed] [Google Scholar]
- Geldard F. A., Sherrick C. E. The cutaneous "rabbit": a perceptual illusion. Science. 1972 Oct 13;178(4057):178–179. doi: 10.1126/science.178.4057.178. [DOI] [PubMed] [Google Scholar]
- Gordon G., Manson J. R. Cutaneous receptive fields of single nerve cells in the thalamus of the cat. Nature. 1967 Aug 5;215(5101):597–599. doi: 10.1038/215597a0. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch H. V., Spinelli D. N. Modification of the distribution of receptive field orientation in cats by selective visual exposure during development. Exp Brain Res. 1971 Jun 29;12(5):509–527. doi: 10.1007/BF00234246. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvärinen J., Poranen A. Function of the parietal associative area 7 as revealed from cellular discharges in alert monkeys. Brain. 1974 Dec;97(4):673–692. doi: 10.1093/brain/97.1.673. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J., Poranen A. Receptive field integration and submodality convergence in the hand area of the post-central gyrus of the alert monkey. J Physiol. 1978 Oct;283:539–556. doi: 10.1113/jphysiol.1978.sp012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvilehto T., Hämäläinen H., Laurinen P. Characteristics of single mechanoreceptive fibres innervating hairy skin of the human hand. Exp Brain Res. 1976 May 10;25(1):45–61. doi: 10.1007/BF00237325. [DOI] [PubMed] [Google Scholar]
- Knibestöl M., Vallbo A. B. Single unit analysis of mechanoreceptor activity from the human glabrous skin. Acta Physiol Scand. 1970 Oct;80(2):178–195. doi: 10.1111/j.1748-1716.1970.tb04783.x. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Michael C. R. Receptive fields of single optic nerve fibers in a mammal with an all-cone retina. II: directionally selective units. J Neurophysiol. 1968 Mar;31(2):257–267. doi: 10.1152/jn.1968.31.2.257. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B., Lynch J. C., Georgopoulos A., Sakata H., Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol. 1975 Jul;38(4):871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B., Talbot W. H., Sakata H., Hyvärinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969 May;32(3):452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- Pubols L. M., Leroy R. F. Orientation detectors in the primary somatosensory neocortex of the raccoon. Brain Res. 1977 Jun 24;129(1):61–74. doi: 10.1016/0006-8993(77)90970-2. [DOI] [PubMed] [Google Scholar]
- Randolph M., Semmes J. Behavioral consequences of selective subtotal ablations in the postcentral gyrus of Macaca mulatta. Brain Res. 1974 Apr 12;70(1):55–70. doi: 10.1016/0006-8993(74)90211-x. [DOI] [PubMed] [Google Scholar]
- Sakata H., Takaoka Y., Kawarasaki A., Shibutani H. Somatosensory properties of neurons in the superior parietal cortex (area 5) of the rhesus monkey. Brain Res. 1973 Dec 21;64:85–102. doi: 10.1016/0006-8993(73)90172-8. [DOI] [PubMed] [Google Scholar]
- Schwarz D. W., Fredrickson J. M. Tactile direction sensitivity of area 2 oral neurons in the rhesus monkey cortex. Brain Res. 1971 Apr 2;27(2):397–401. doi: 10.1016/0006-8993(71)90270-8. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Whitsel B. L., Roppolo J. R., Werner G. Cortical information processing of stimulus motion on primate skin. J Neurophysiol. 1972 Sep;35(5):691–717. doi: 10.1152/jn.1972.35.5.691. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965 Nov;28(6):1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- Wolbarsht M. L., Macnichol E. F., Jr, Wagner H. G. Glass Insulated Platinum Microelectrode. Science. 1960 Nov 4;132(3436):1309–1310. doi: 10.1126/science.132.3436.1309. [DOI] [PubMed] [Google Scholar]


