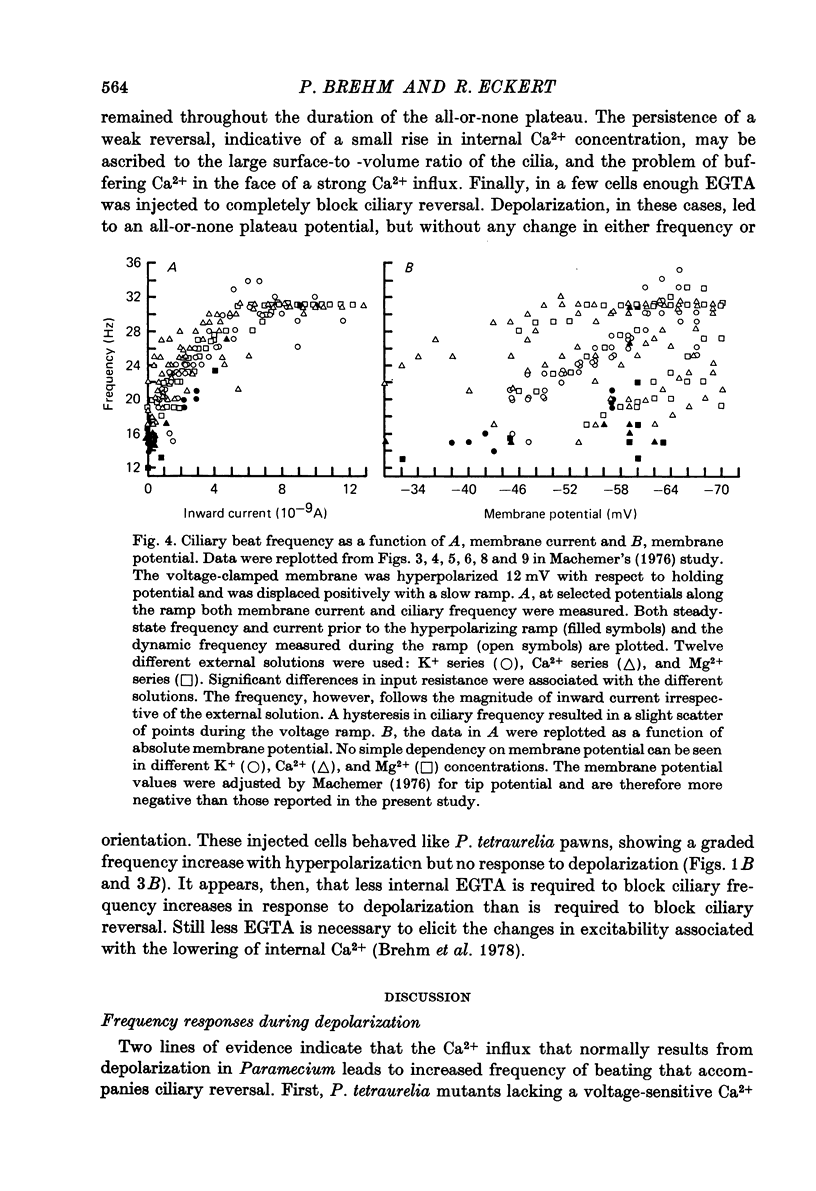
Abstract
1. The role of the surface membrane in the control of ciliary beat frequency in Paramecium was examined by intracellular electrophysiological techniques and pressure injection of Ca2+ and EGTA. Experiments were done on wild type P. caudatum and on both the wild type and a pawn mutant of P. tetraurelia. 2. The increased frequency of beating that accompanies reversal of power stroke orientation in response to depolarization in the wild type fails to occur during depolarization in the mutant pawn, which also fails to exhibit ciliary reversal upon depolarization. 3. Injection of moderate amounts of EGTA blocked the frequency increase without interfering with reversal of the beat in response to depolarization of the wild type. Larger injection of EGTA also prevented reversed beating. 4. The beat frequency in the normal (forward-swimming) direction increased during hyperpolarization in pawn. The hyperpolarizing frequency-voltage relations were quantitatively similar to those of the wild type. 5. Injection of EGTA to a final concentration of 10 mM into wild type cells neither modified the resting frequency nor blocked the frequency increase which normally accompanies hyperpolarization. 6. Transient ciliary reversal in both pawn and wild type produced by injection of Ca2+ could be terminated by the passage of inward current. The power stroke returned to the normal forward-swimming direction and the ciliary beating frequency increased. Upon termination of the inward current the cilia of Ca2+-injected cells again beat in reverse for many seconds. 7. The results support previous reports that increased frequency of beating and ciliary reversal seen in response to depolarization both require the entry of Ca2+ through the surface membrane. On the other hand, the results indicate that frequency increase with hyperpolarization is independent of an altered rate of Ca2+ entry. 8. Increased frequency during hyperpolarization appears to be related more closely to electrotonic membrane current than to membrane potential. It is proposed that inward current might activate high frequency beating by altering the ionic environment of the axoneme within the restricted volume of the cilium by electrophoretic means.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brehm P., Dunlap K., Eckert R. Calcium-dependent repolarization in Paramecium. J Physiol. 1978 Jan;274:639–654. doi: 10.1113/jphysiol.1978.sp012171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Localization of calcium channels in Paramecium caudatum. J Physiol. 1977 Sep;271(1):119–133. doi: 10.1113/jphysiol.1977.sp011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Eckert R., Murakami A. Calcium dependence of ciliary activity in the oviduct of the salamander Necturus. J Physiol. 1972 Nov;226(3):699–711. doi: 10.1113/jphysiol.1972.sp010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Naitoh Y., Friedman K. Sensory mechanisms in Paramecium. I. Two components of the electric response to mechanical stimulation of the anterior surface. J Exp Biol. 1972 Jun;56(3):683–694. doi: 10.1242/jeb.56.3.683. [DOI] [PubMed] [Google Scholar]
- Eckert R., Naitoh Y. Passive electrical properties of Paramecium and problems of ciliary coordination. J Gen Physiol. 1970 Apr;55(4):467–483. doi: 10.1085/jgp.55.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F., Nuccitelli R. Electrical controls of development. Annu Rev Biophys Bioeng. 1977;6:445–476. doi: 10.1146/annurev.bb.06.060177.002305. [DOI] [PubMed] [Google Scholar]
- KINOSITA H. Electrophoretic theory of pigment migration within fish melanophore. Ann N Y Acad Sci. 1963 Feb 15;100:992–1004. [PubMed] [Google Scholar]
- Kung C., Eckert R. Genetic modification of electric properties in an excitable membrane (paramecium-calcium conductance-electrophysiological measurements-membrane mutant). Proc Natl Acad Sci U S A. 1972 Jan;69(1):93–97. doi: 10.1073/pnas.69.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Kaneko H. Reactivated triton-extracted models o paramecium: modification of ciliary movement by calcium ions. Science. 1972 May 5;176(4034):523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Calcium ion distribution in cytoplasm visualised by aequorin: diffusion in cytosol restricted by energized sequestering. Science. 1975 Dec 19;190(4220):1204–1206. doi: 10.1126/science.1198106. [DOI] [PubMed] [Google Scholar]
- Satow Y., Kung C. Mutants with reduced Ca activation in Paramecium aurelia. J Membr Biol. 1976 Aug 26;28(2-3):277–294. doi: 10.1007/BF01869701. [DOI] [PubMed] [Google Scholar]
- Schein S. J., Bennett M. V., Katz G. M. Altered calcium conductance in pawns, behavioural mutants of Paramecium aurelia. J Exp Biol. 1976 Dec;65(3):699–724. doi: 10.1242/jeb.65.3.699. [DOI] [PubMed] [Google Scholar]
- Woodruff R. I., Telfer W. H. Polarized intercellular bridges in ovarian follicles of the cecropia moth. J Cell Biol. 1973 Jul;58(1):172–188. doi: 10.1083/jcb.58.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]


